SUPPLEMENT FACTS
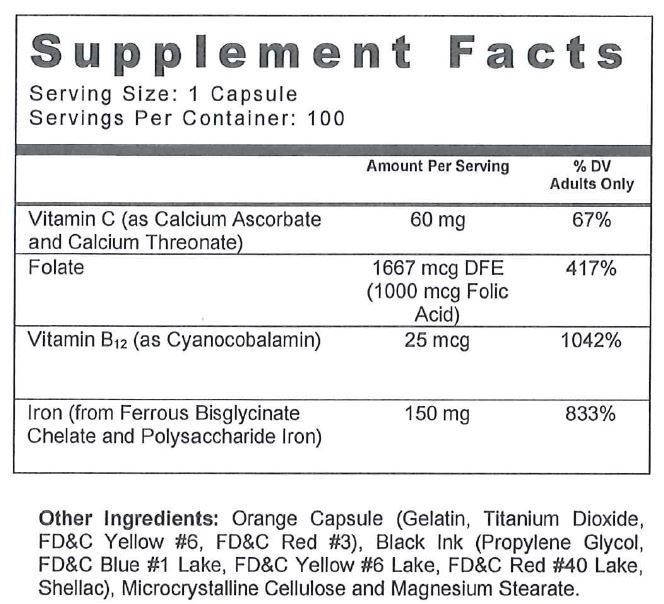
Taron Forte Multivitamin/Mineral with Iron capsules are indicated for the distinctive nutritional requirements of persons being treated for iron deficiencies by a physician.
CONTRAINDICATIONS
Taron Forte Capsules are contraindicated in patients with a known hypersensitivity to any of the components of this product.
Hemochromatosis and hemosiderosis are contraindications to iron therapy.
WARNINGS
| WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately. |
PRECAUTIONS
General: The type of anemia and the underlying cause or causes should be determined before starting therapy with Taron Forte Capsules. Since the anemia may be a result of a systemic disturbance, such as recurrent blood loss, the underlying cause or causes should be corrected, if possible.
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where B12 is deficient.
Folic acid in doses above 0.1 mg daily may obscure pernicious anemia assessment, such that hematologic remission can occur while neurological manifestations remain progressive.
Pediatric Use: Safety and effectiveness of this product have not been established in pediatric patients.
ADVERSE REACTIONS
Adverse reactions with iron therapy may include constipation, diarrhea, nausea, vomiting, dark stools, and abdominal pain. Adverse reactions with iron therapy are usually transient. Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
The clinical cause of acute iron overdosage can be variable. Initial symptoms may include: abdominal pain, nausea, vomiting, diarrhea, tarry stools, melena, hematemesis, hypotension, tachycardia, metabolic acidosis, hyperglycemia, dehydration, drowsiness, pallor, cyanosis, lassitude, seizures, shock, and coma.
HOW SUPPLIED
Taron Forte Capsules are packaged in child-resistant bottles of 100 capsules.
PRODUCT CODE 13811-042-10.
STORAGE
Store at 20°-25°C (68°-77°F), excursion permitted to 15°-30°C (59°-86°F) [See USP Controlled Room Temperature]. Protect from light and moisture. Dispense in a tight, light-resistant container with a child-resistant closure as defined in the USP/NF.
KEEP OUT OF REACH OF CHILDREN.
For use on the order of a healthcare practitioner.
Call your doctor about side effects. To report side effects, call Trigen Laboratories at 1-770-509-4500 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
PLR-TARONFORTE-00001-1 Rev. 12/2021
Manufactured for:
Trigen Laboratories, LLC
Alpharetta, GA 30005

