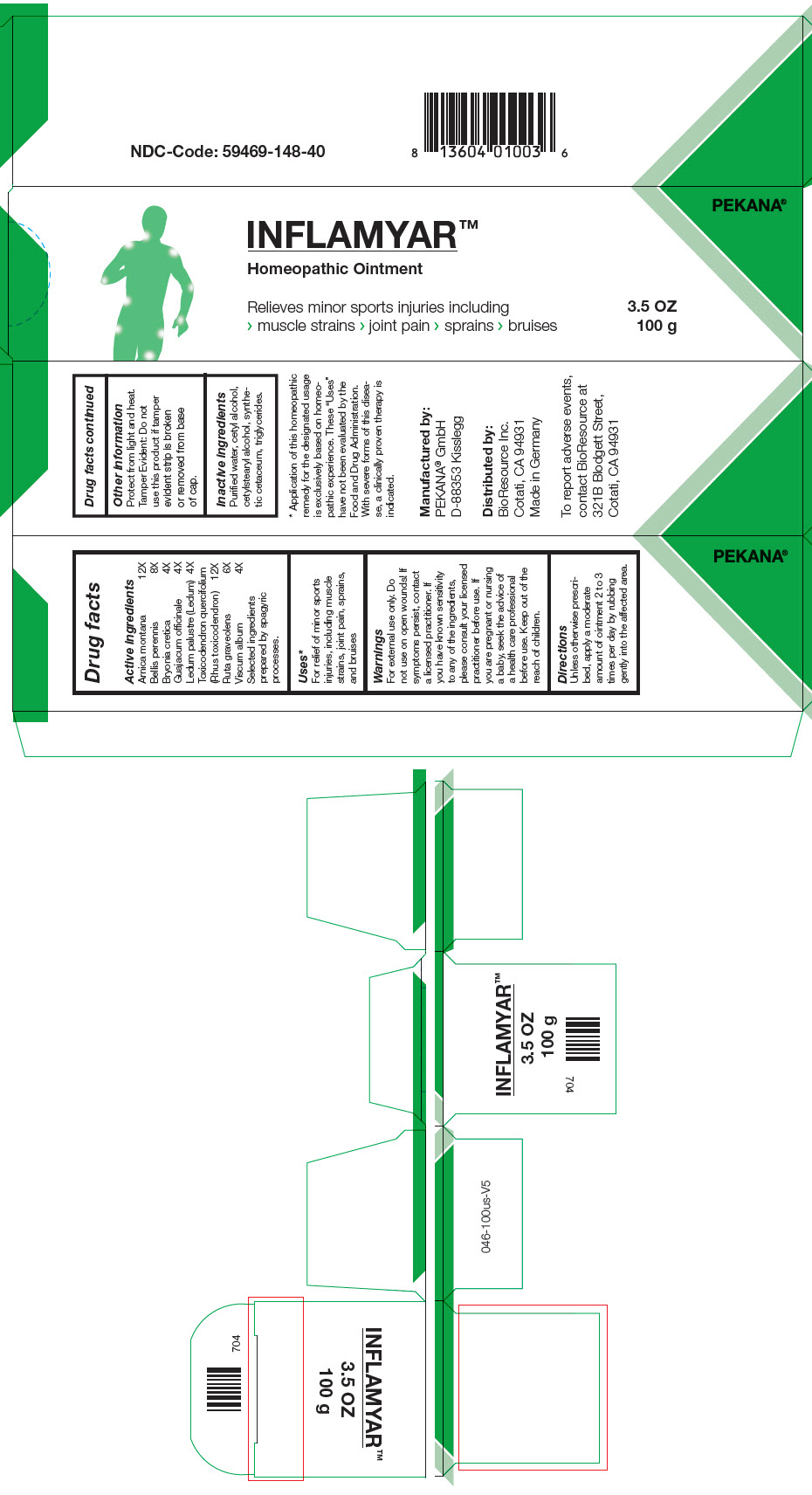
INFLAMYAR- arnica montana root, bryonia dioica root, guaiacum officinale resin, toxicodendron pubescens shoot, bellis perennis, ledum palustre twig, ruta graveolens flowering top, and viscum album fruiting top ointment
PEKANA Naturheilmittel GmbH
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
| Active ingredients | |
| Arnica montana | 12X |
| Bellis perennis | 8X |
| Bryonia cretica | 4X |
| Guajacum officinale | 4X |
| Ledum palustre (Ledum) | 4X |
| Toxicodendron quercifolium (Rhus toxicodendron) | 12X |
| Ruta graveolens | 6X |
| Viscum album | 4X |
Selected ingredients prepared by spagyric processes.
Uses1
For relief of minor sports injuries, including muscle strains, joint pain, sprains, and bruises
Warnings
For external use only. Do not use on open wounds! If symptoms persist, contact a licensed practitioner. If you have known sensitivity to any of the ingredients, please consult your licensed practitioner before use. If you are pregnant or nursing a baby, seek the advice of a health care professional before use.
Keep out of the reach of children.
Directions
Unless otherwise prescribed, apply a moderate amount of ointment 2 to 3 times per day by rubbing gently into the affected area.
Other information
Protect from light and heat.
Tamper Evident
Do not use this product if tamper evident strip is broken or removed from base of cap.
Inactive ingredients
Purified water, cetyl alcohol, cetylstearyl alcohol, synthetic cetaceum, triglycerides.
Manufactured by:
PEKANA® GmbH
D-88353 Kisslegg
Distributed by:
BioResource Inc.
Cotati, CA 94931
PRINCIPAL DISPLAY PANEL - 100 g Tube Box
INFLAMYAR™
Homeopathic Ointment
Relieves minor sports injuries including
> muscle strains > joint pain > sprains > bruises
3.5 OZ
100 g
PEKANA®