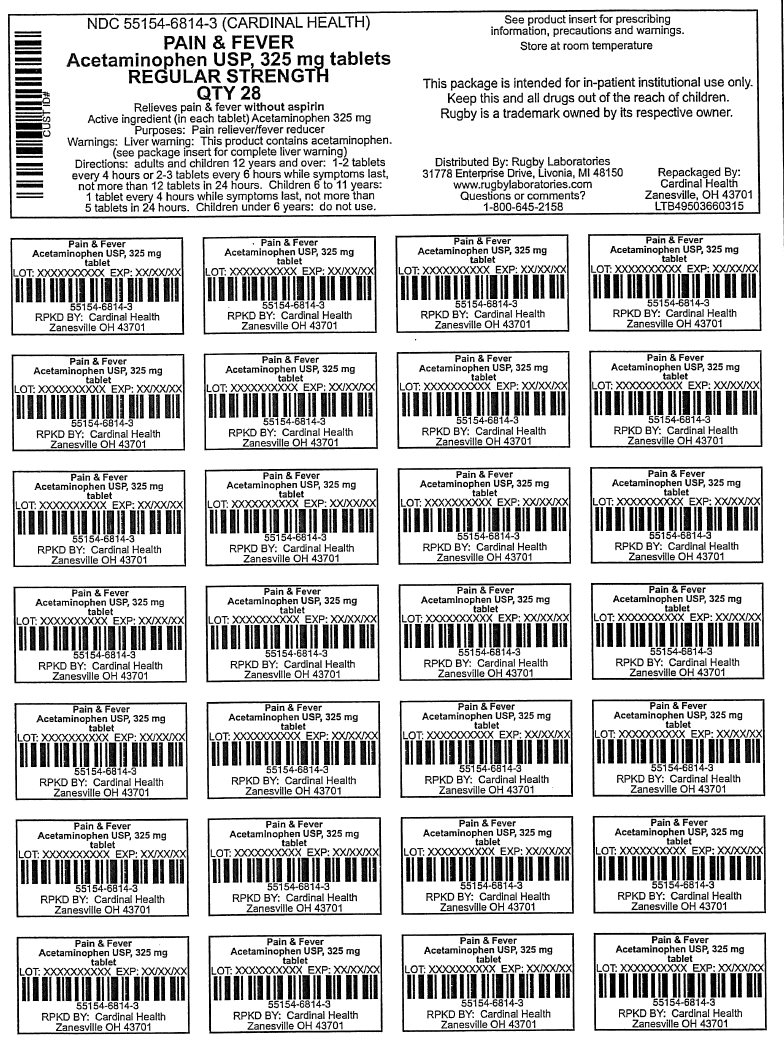
PAIN AND FEVER- acetaminophen tablet
Cardinal Health
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Uses
temporarily reduces fever and relives minor aches and pains caused by
- •
- Headache
- •
- muscular aches
- •
- common cold
- •
- toothache
- •
- premenstrual and menstrual cramps
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if
- •
- adult takes more than 12 tablets in 24 hours,which is the maximum daily amount
- •
- child takes more than 5 tablets in 24 hours, which is the maximum daily amount
- •
- taken with other drugs containing acetaminophen
- •
- adult has 3 or more alcoholic drinks every day while using this product
Do not use
with any other drug containing acetaminophen (prescription or non prescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Directions
|
Adults and children 12 years and over |
1-2 tablets every 4 hours or 2-3 tablets every 6 hours while symptoms last, not more than 12 tablets in 24 hours. |
|
Children 6 to 11 years |
1 tablet every 4 hours while symptoms last, not more than 5 tablets in 24 hours. |
|
Children under 6 years |
do not use |
Questions or comments?
call 1-800-645-2158
COMPARE TO ACTIVE INGREDIENT IN REGULAR STRENGTH TYLENOLR*
*Rugby Laboratories, Inc. is not affiliated with the owner of the trademark TylenolR.
www.rugbylaboratories.com
Distributed By: Rubgy Laboratories
31778 Enterprise Drive, Livonia, MI 48150
Rev. 04/13
IT48470180215
Cardinal Health
Zanesville, OH 43701
| PAIN AND FEVER
acetaminophen tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Cardinal Health (188557102) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cardinal Health | 188557102 | REPACK(55154-6814) | |