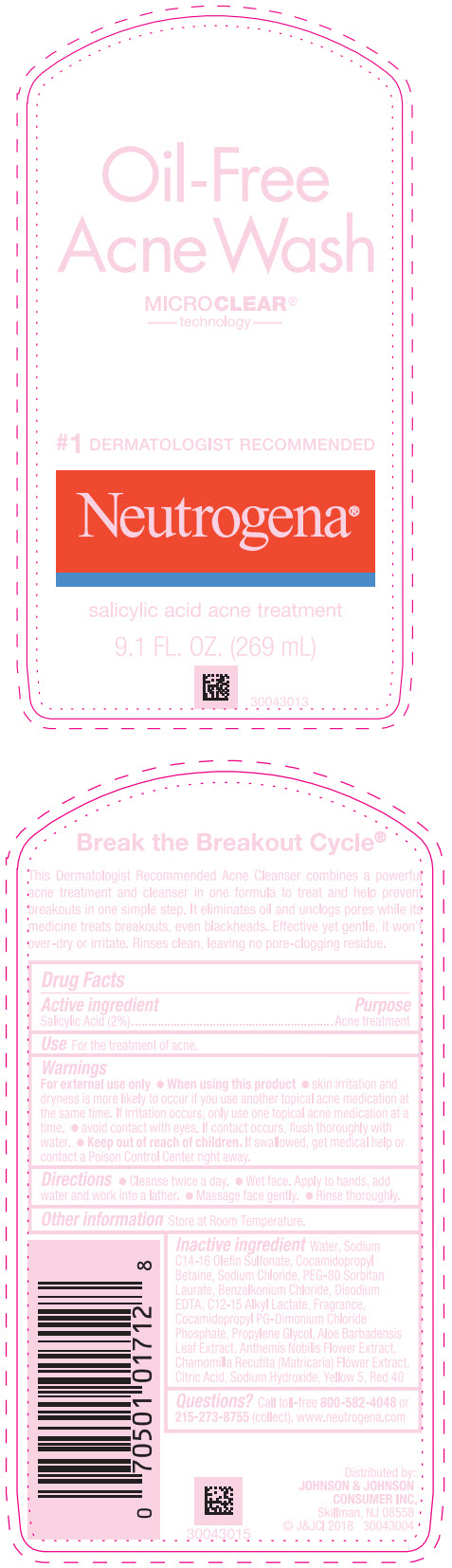
NEUTROGENA OIL FREE ACNE WASH- salicylic acid lotion
Johnson & Johnson Consumer Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Salicylic Acid 2%
Use
For the treatment of acne.
Warnings
For external use only.
When using this product
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- avoid contact with eyes. If contact occurs, flush thoroughly with water.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Cleanse twice a day.
- Wet face. Apply to hands, add water and work into a lather.
- Massage face gently.
- Rinse thoroughly.
Other information
Store at Room Temperature.
Inactive ingredients
Water, Sodium C14-16 Olefin Sulfonate, Cocamidopropyl Betaine, Sodium Chloride, PEG-80 Sorbitan Laurate, Benzalkonium Chloride, Disodium EDTA, C12-15 Alkyl Lactate, Fragrance, Cocamidopropyl PG-Dimonium Chloride Phosphate, Propylene Glycol, Aloe Barbadensis Leaf Extract, Anthemis Nobilis Flower Extract, Chamomilla Recutita (Matricaria) Flower Extract, Citric Acid, Sodium Hydroxide, Yellow 5, Red 40
Questions?
Call toll-free
800-582-4048 or
215-273-8755 (collect). www.neutrogena.com
Distributed by:
JOHNSON & JOHNSON
CONSUMER INC.
Skillman, NJ 08558
PRINCIPAL DISPLAY PANEL - 269 mL Bottle Label
Oil-Free
Acne Wash
MICRO
CLEAR®
technology
# 1 DERMATOLOGIST RECOMMENDED
Neutrogena
®
salicylic acid acne treatment
9.1 FL. OZ. (269 mL)
Johnson & Johnson Consumer Inc.