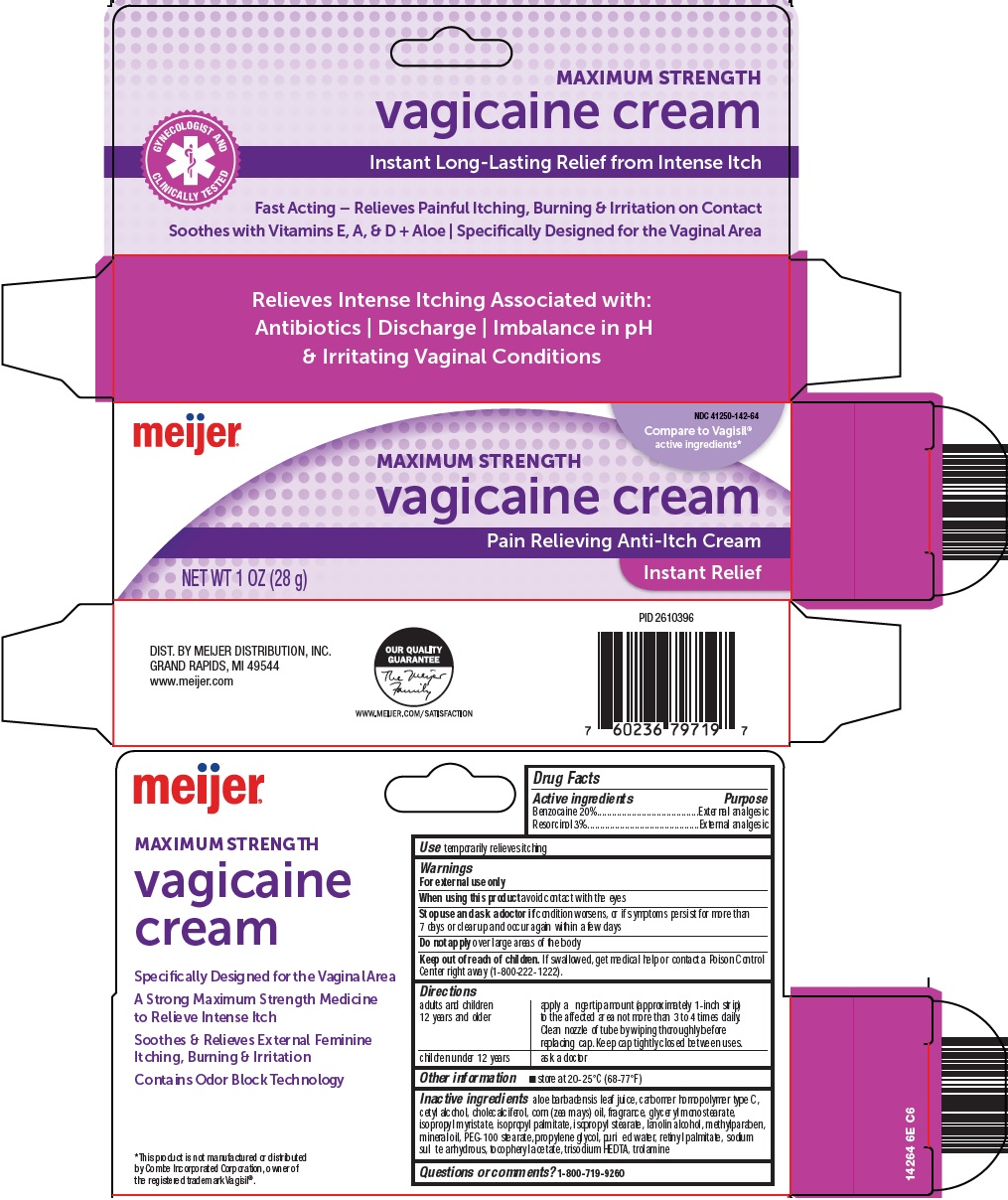
Warnings
For external use only
Directions
|
adults and children 12 years and older |
apply a fingertip amount (approximately 1-inch strip) to the affected area not more than 3 to 4 times daily. Clean nozzle of tube by wiping thoroughly before replacing cap. Keep cap tightly closed between uses. |
|
children under 12 years |
ask a doctor |
Inactive ingredients
aloe barbadensis leaf juice, carbomer homopolymer type C, cetyl alcohol, cholecalciferol, corn (zea mays) oil, fragrance, glyceryl monostearate, isopropyl myristate, isopropyl palmitate, isopropyl stearate, lanolin alcohol, methylparaben, mineral oil, PEG-100 stearate, propylene glycol, purified water, retinyl palmitate, sodium sulfite anhydrous, tocopheryl acetate, trisodium HEDTA, trolamine
Principal Display Panel
MAXIMUM STRENGTH
vagicaine cream
GYNECOLOGIST AND CLINICALLY TESTED
Instant Long-Lasting Relief from Intense Itch
Fast Acting – Relieves Painful Itching, Burning & Irritation on Contact
Soothes with Viatmins E, A, & D + Aloe | Specifically Designed for the Vaginal Area
Compare to Vagisil® active ingredients
MAXIMUM STRENGTH
vagicaine cream
Pain Relieving Anti-Itch Cream
NET WT. 1 OZ (28 g)
Instant Relief