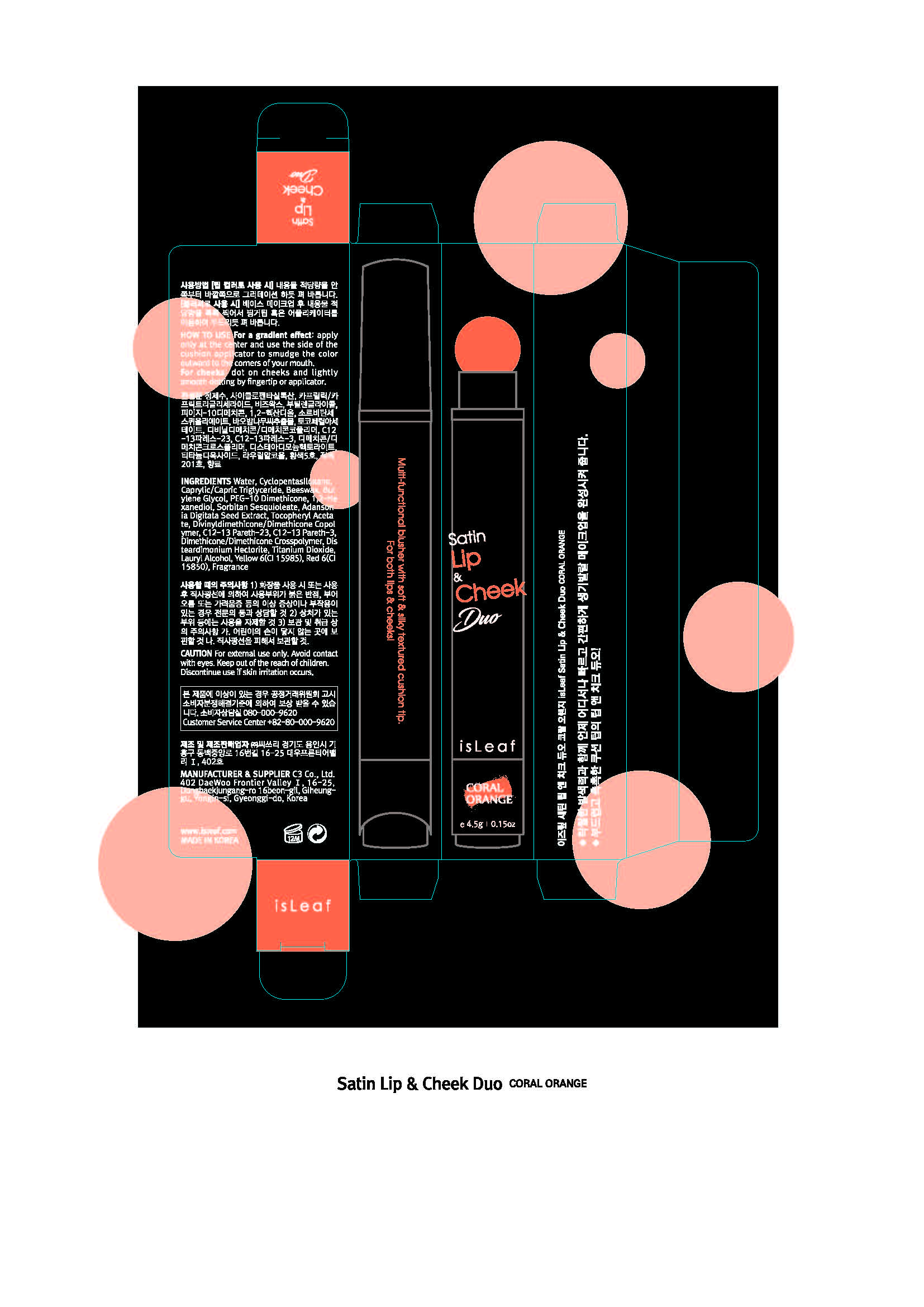
For a gradient effect, apply only at the center and use the side of the cushion applicator to smudge the color outward to the corners of your mouth.
For cheeks, dot on cheeks and lightly smooth dotting by fingertip or applicator
1. If the following symptoms occur after product use, stop using the product immediately and consult a dermatologist (continuous use can exacerbate the symptoms).
1) Occurrence of red spots, swelling, itchiness, and other skin irritation
2) If the symptoms above occur after the application area is exposed to direct sunlight
2. Do not use on open wounds, eczema, and other skin irritations
3. Precaution for Storage and Handling
1) Close the lid after use
2) Keep out of reach of infants and children
3) Do not to store in a place with high/low temperature and exposed to direct sunlight
4. Use as avoiding eye areas.