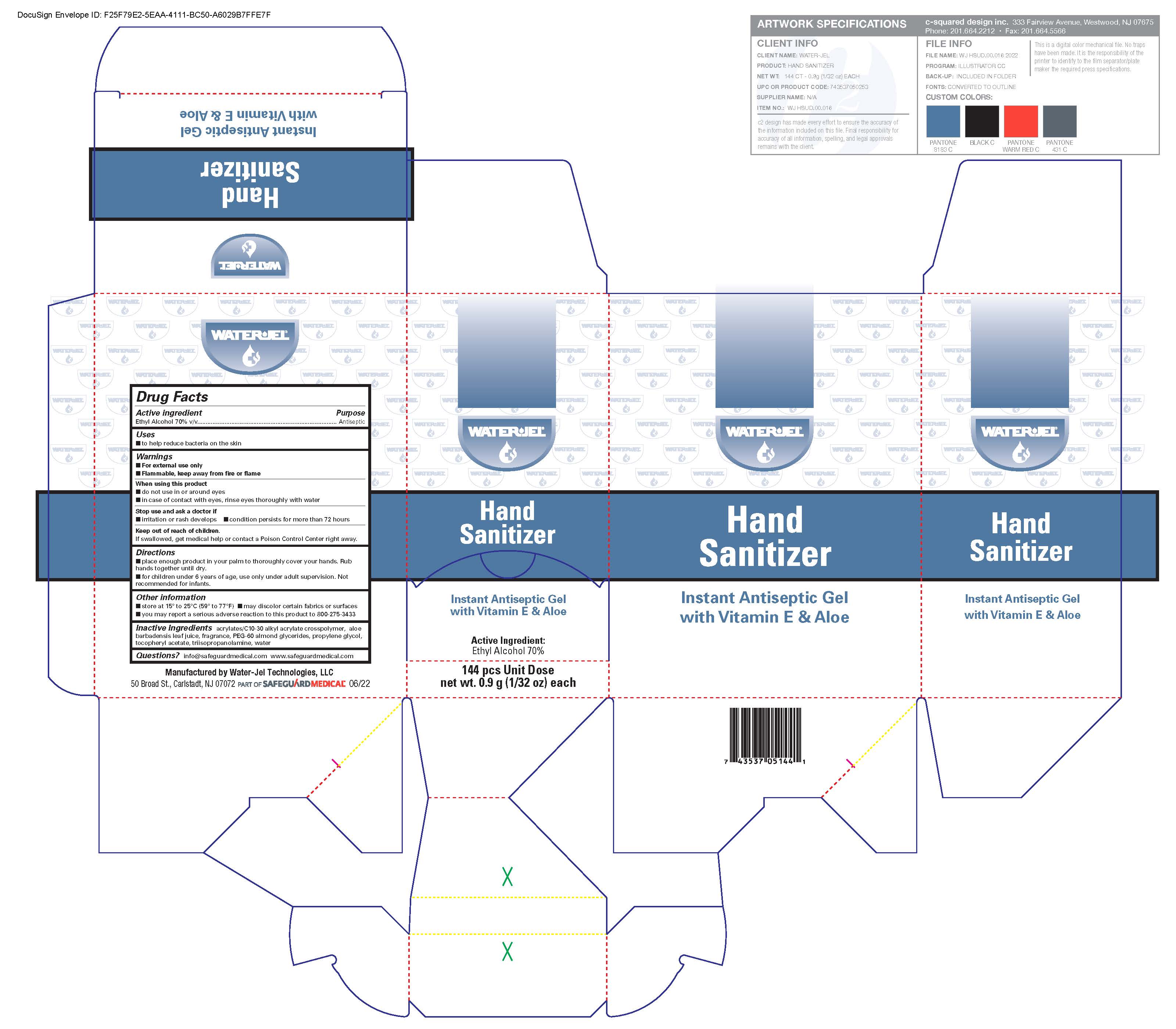
INSTANT HAND SANITIZER- alcohol liquid
Water-Jel Technologies
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Ethyl alcohol 70% v/v
Uses
- to help reduce bacteria on the skin
Warnings
For external use only
Flammable, keep away from fire or flame
When using this product
- do not use in the eyes
- discontinue use if irritation and redness develops. If condition persists for more than 72 hours consult a doctor.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
- store at 15
0 to 25
0 C (59
0 to 77
0 F)
- may discolor certain fabrics or surfaces
- you may report a serious adverse reaction to this product to 800-275-3433
Inactive ingredients
acrylates/C10-30 alkyl acrylate crosspolymer, aloe barbadensis leaf juice, dl-alpha tocopheryl acetate, fragrance, PEG-60 almond glycerides, propylene glycol, tocopheryl acetate, purified water, triisopropanolamine
Questions
info@safeguardmedical.com www.safeguardmedical.com
Directions
- place enough product in your palm to thoroughly cover your hands. Rub hands together until dry.
- for children under 6 years of age, use only under adult supervision. Not recommended for infants.
Principal Display Panel
Enter section text here