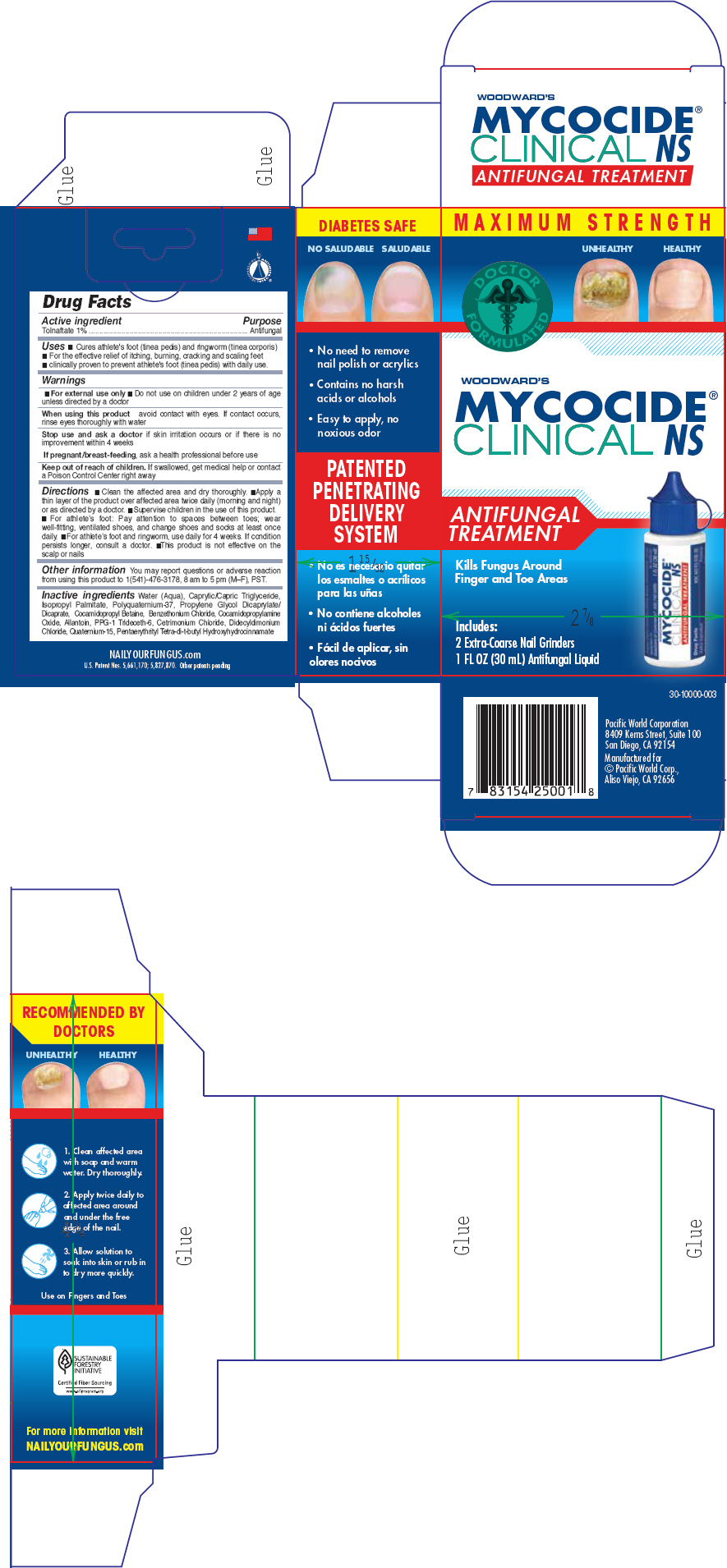
Uses
- Cures most fungal skin infections, including athlete's foot (tinea pedis) and ringworm (tinea corporis)
- Effectively relieves itching, burning, cracking and scaling accompanying such conditions
Warnings
For external use only.
Do not use
- near the mouth or eyes
- with known sensitivities to any listed ingredients
- on children under 2 years of age unless directed by a doctor
When using this product avoid eye contact. If accidental eye contact occurs, rinse thoroughly with water for 10-15 minutes.
Directions
- Clean affected area with soap and warm water and dry thoroughly
- Apply a thin layer two times a day (mornings and evenings) to affected area especially the space between and around toes
- For athlete's foot, use daily for four weeks or as directed by a physician
- Allow solution to soak into skin or rub in to dry more quickly before putting on socks
- This product is not effective on scalp or nails.
- Following a proper foot hygiene regimen along with wearing well fitting, ventilated shoes and clean socks that are changed at least daily is helpful in preventing future infections
Other information
You may report questions or adverse reaction from using this product to 1(541)-476-3178, 8 am to 5 pm (M–F), PST.
Inactive ingredients
Water (Aqua), Caprylic/Capric Triglycerides, Isopropyl Palmitate, Polyquaternium 37, Propylene Glycol, Dicaprylate Dicaprate, PPG-1 Trideceth-6, Cocamidopropyl Betaine, Cocamidopropylamine Oxide, Phemerol Chloride, Cetrimonium Chloride, Allantoin, Didecyldimonium Chloride, Quaternium-15.