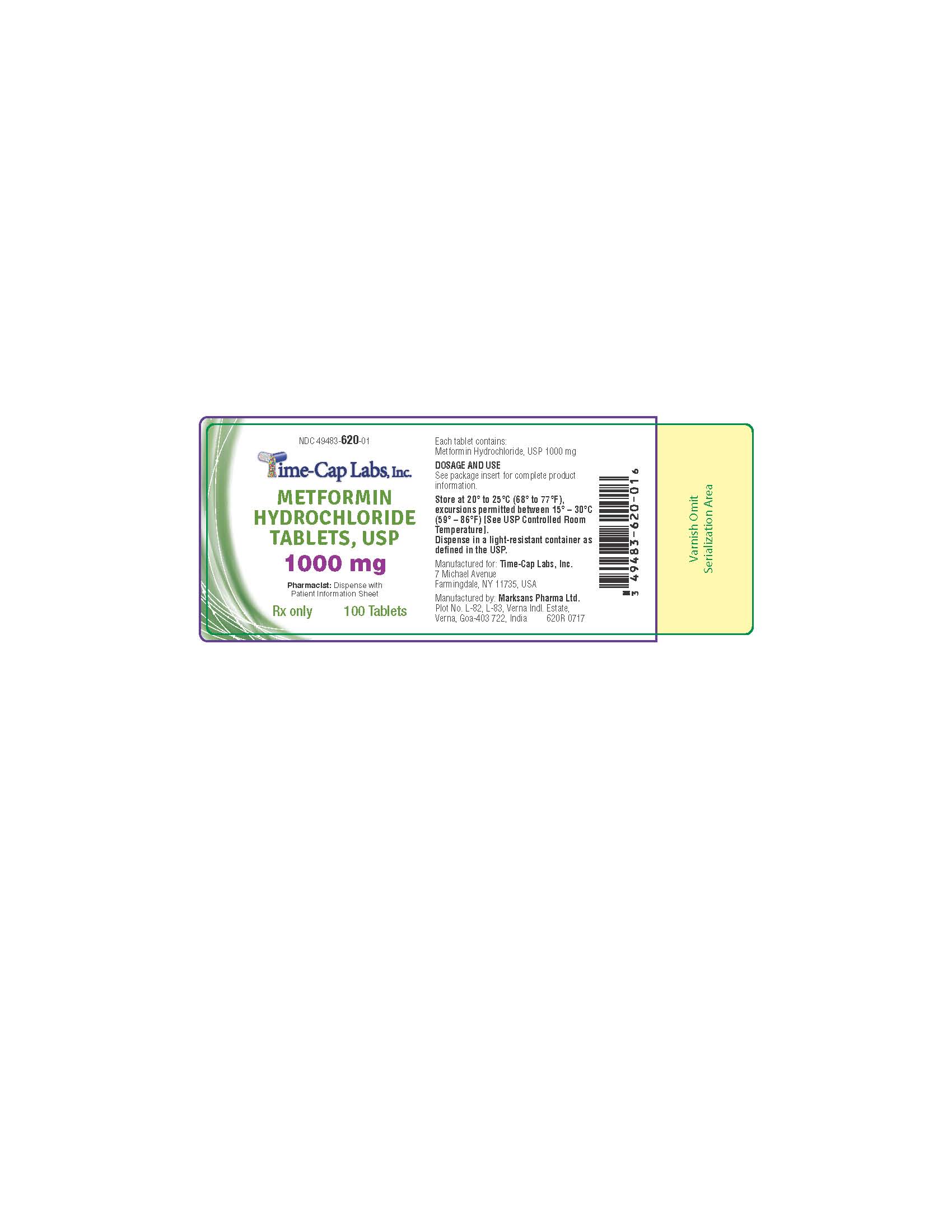
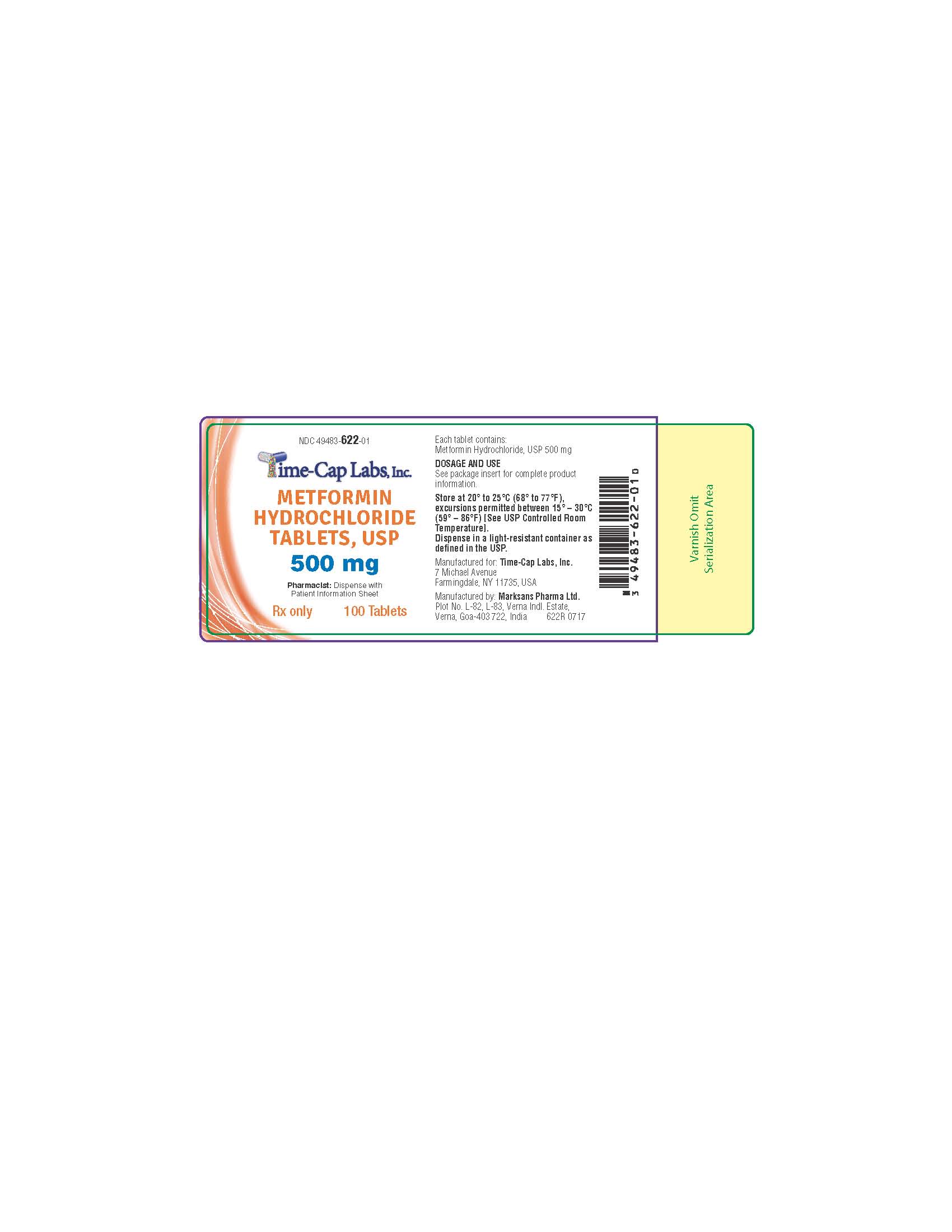
HOW SUPPLIED
METFORMIN HYDROCHLORIDE TABLETS, USP 500 MG ARE WHITE TO OFF-WHITE COLORED, FILM COATED, ROUND SHAPED WITH BEVELED EDGES ON BOTH SIDES AND DEBOSSED WITH "134" ON ONE SIDE AND PLAIN ON THE OTHER SIDE. BOTTLES OF 100 AND 500.
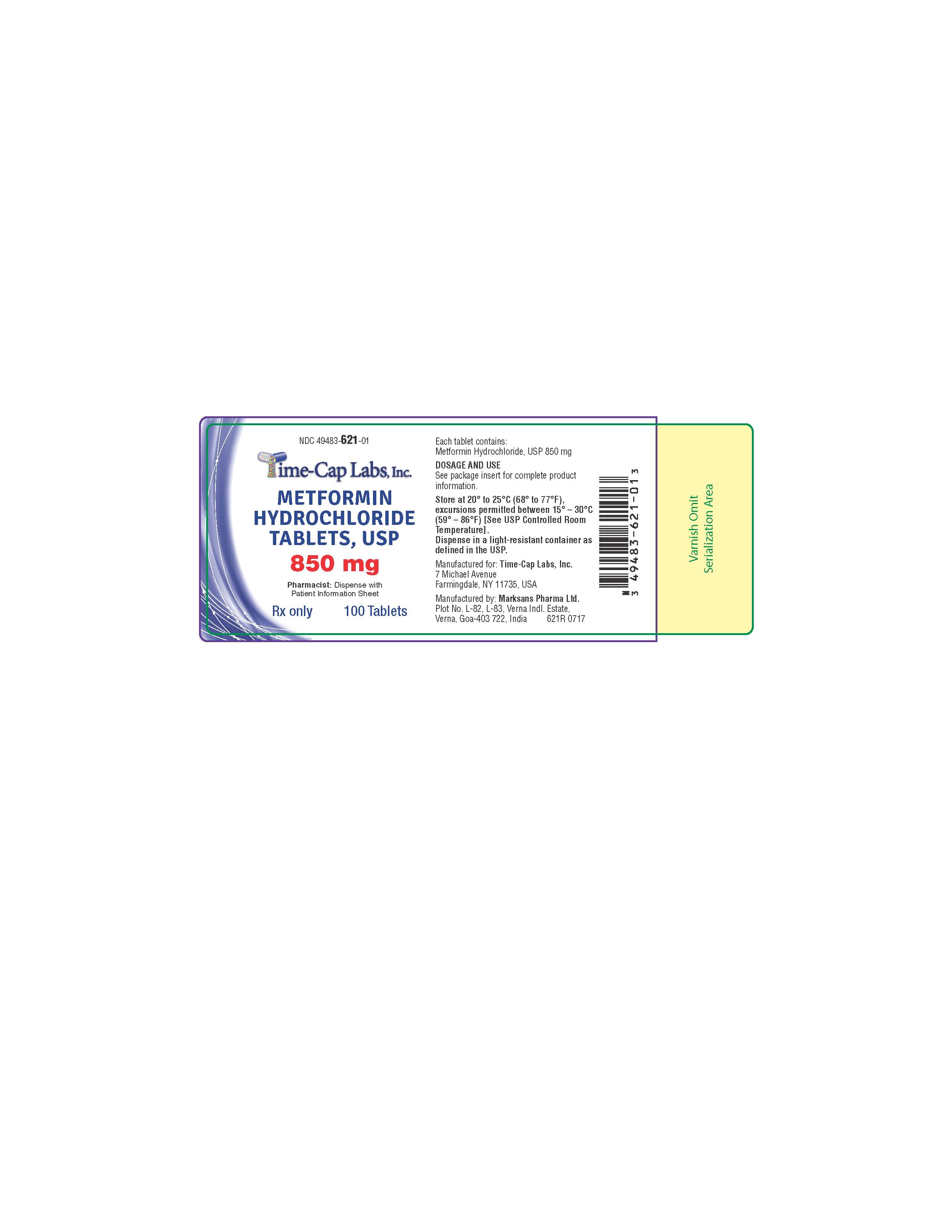
HOW SUPPLIED
METFORMIN HYDROCHLORIDE TABLETS, USP 850 MG ARE WHITE TO OFF-WHITE COLORED, FILM COATED, ROUND SHAPED WITH BEVELED EDGES ON BOTH SIDES AND DEBOSSED WITH "131" ON ONE SIDE AND PLAIN ON THE OTHER SIDE. BOTTLES OF 100 AND 500.