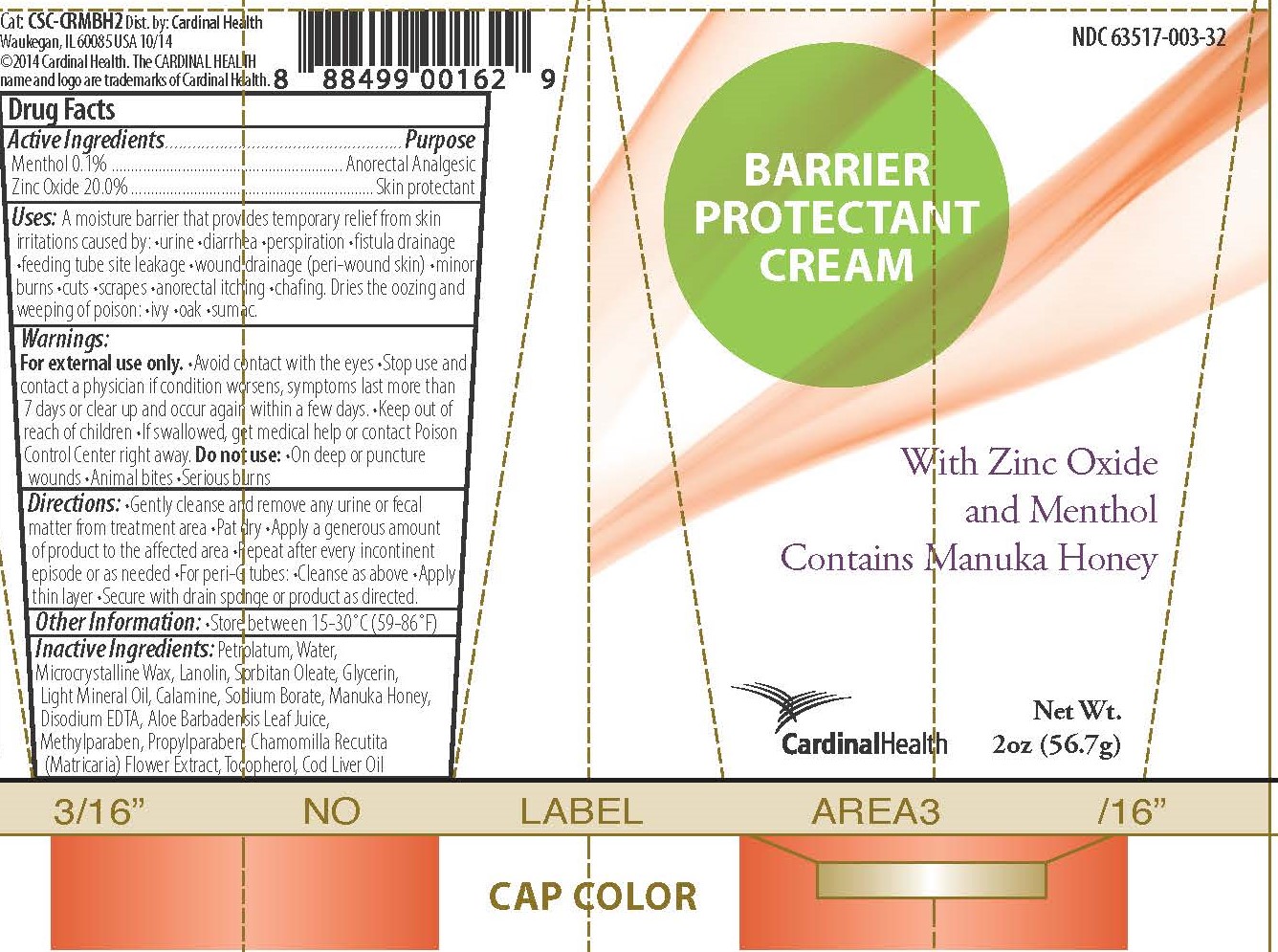
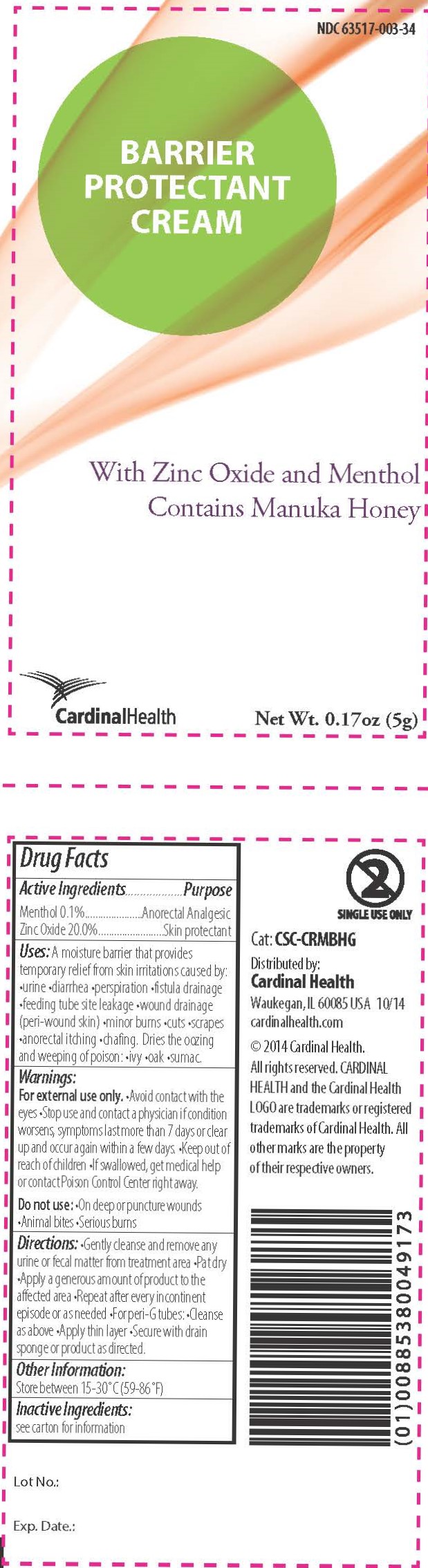
Uses
A moisture barrier that provides temporary relief from skin irritations caused by •urine •diarrhea •perspiration •fistula drainage •feeding tube site leakage •wound drainage (peri-wound skin) •minor burns •cuts •scrapes •anorectal itching •chafing
Dries the oozing and weeping of poison •ivy •oak •sumac
Warnings
For external use only.
- Avoid contact with the eyes
- Stop use and contact a physician if condition worsens, symptoms last more than 7 days, or clear up and occur again within a few days.
- Keep out of reach of children
- If swallowed, get medical help or contact Poison Control Center right away.
Do not use:
- On deep or puncture wounds
- Animal bites
- Serious burns
Directions
- Gently cleanse and remove any urine or fecal matter from the treatment area
- Pat dry
- Apply a generous amount of product to the affected area
- Repeat after every incontinent episode or as needed
- For peri-G tubes: •Cleanse as above •Apply thin layer •Secure with drain sponge or product as directed.