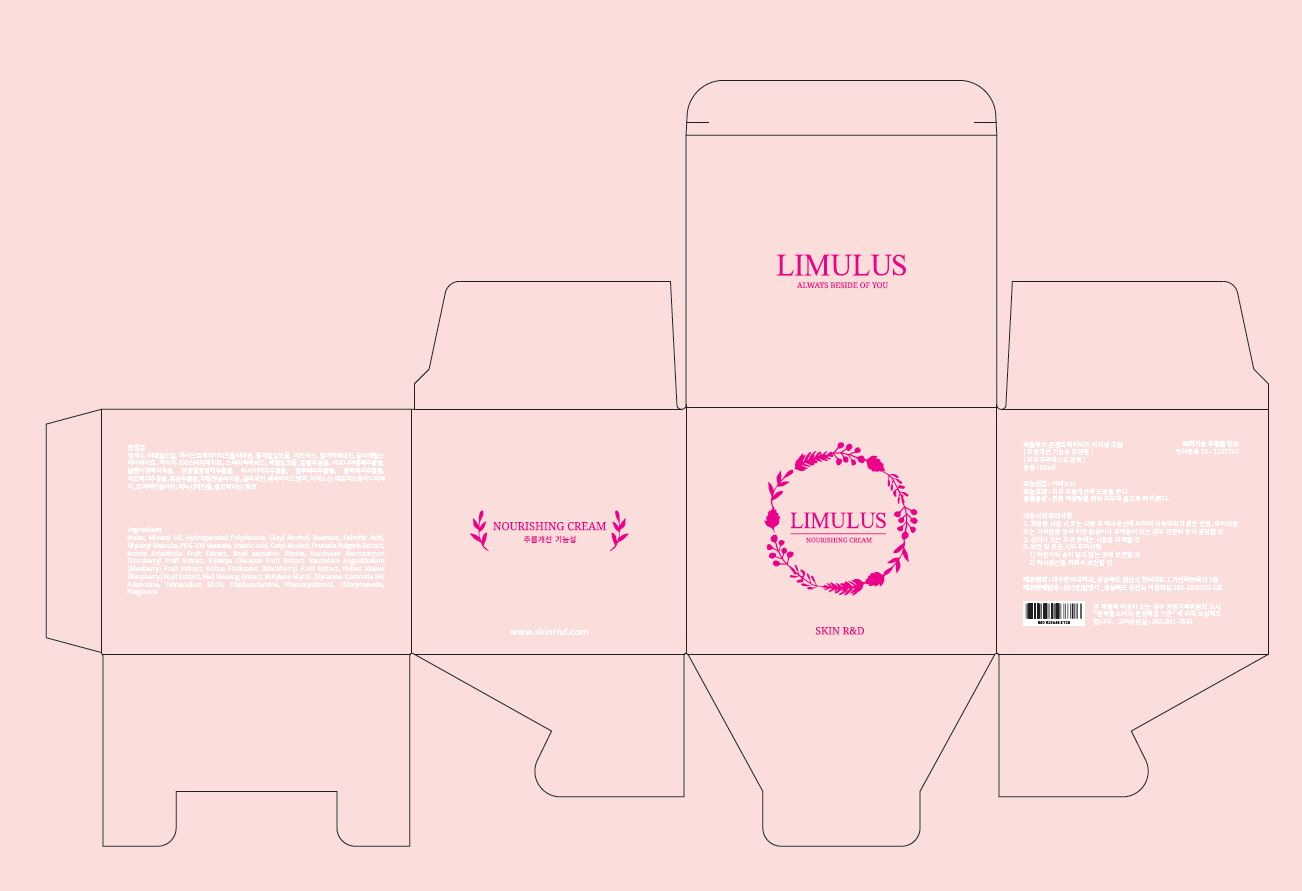
Water
Mineral Oil
Hydrogenated Polydecene
Oleyl Alcohol
Stearic acid
Palmitic Acid
Glyceryl Stearate
PEG-100 Stearate
Beeswax
Cetyl Alcohol
Aronia Arbutifolia Fruit Extract
Glycerine
Butylene Glycol
Phenoxyethanol
Prunella Vulgaris Extract
Snail secretion filtrate
Phenoxyethanol
Vaccinium Macrocarpon (Cranberry) Fruit Extract
Euterpe Oleracea Fruit Extract
Vaccinium Angustifolium (Blueberry) Fruit Extract
Rubus Fruticosus (Blackberry) Fruit Extract
Rubus Idaeus (Raspberry) Fruit Extract
Triethanolamine
Phenoxyethanol
Fragrance
Chlorphenesin
Tetrasodium EDTA
Ceramide NP
Red Ginseng Extract
Precautions for Use
1. Consult with a medical specialist if any abnormal symptoms or side effects, such as red spots, swelling, or itching, occur during the use of this product.
2. Do not use the product on wounded areas.
3. Precautions for storage and handling
1) Keep out of reach of children.
2) Store away from direct sunlight.