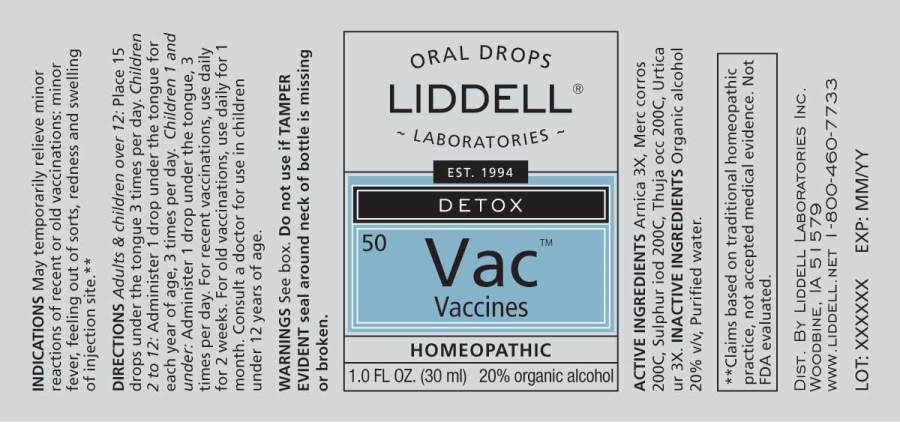
ACTIVE INGREDIENTS:
Arnica Montana 3X, Mercurius Corrosivus 200C, Sulphur Iodatum 200C, Thuja Occidentalis 200C, Urtica Urens 3X
USES:
May temporarily relieve the ill effects - not the therapeutic effects - of oral or injected vaccines. Children and adults may expect temporary relief from symptoms such as:
• minor fever
• pain
• redness
• weakness
• lack of energy
• swelling**
**Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
WARNINGS:
Do not use if you have ever had an allergic reaction to this product or any of its ingredients.
Ask a doctor before use if excessive redness or swelling is present.
Stop use and ask a doctor if symptoms persist, worsen or if new symptoms occur.
Keep out of reach of children. In case of overdose, get medical help or call a Poison Control Center right away.
If pregnant or breast feeding, ask a doctor before using product.
Do not use if TAMPER EVIDENT seal around neck of bottle is missing or broken.
Other Information:
Store at room temperature.
KEEP OUT OF REACH OF CHILDREN:
Keep out of reach of children. In case of overdose, get medical help or call a Poison Control Center right away.
DIRECTIONS:
Adults & children over 12: Place 15 drops under the tongue 3 times per day.
Children 2 to 12: Administer 1 drop under the tongue per year of age, 3 times per day.
Children 1 and under: Administer 1 drop under the tongue, 3 times per day.
For recent vaccinations, use daily for two weeks. For old vaccinations, use daily for one month. Consult a physician for use in children under 12.
INDICATIONS:
May temporarily relieve the ill effects - not the therapeutic effects - of oral or injected vaccines. Children and adults may expect temporary relief from symptoms such as:
• minor fever
• pain
• redness
• weakness
• lack of energy
• swelling**
**Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.