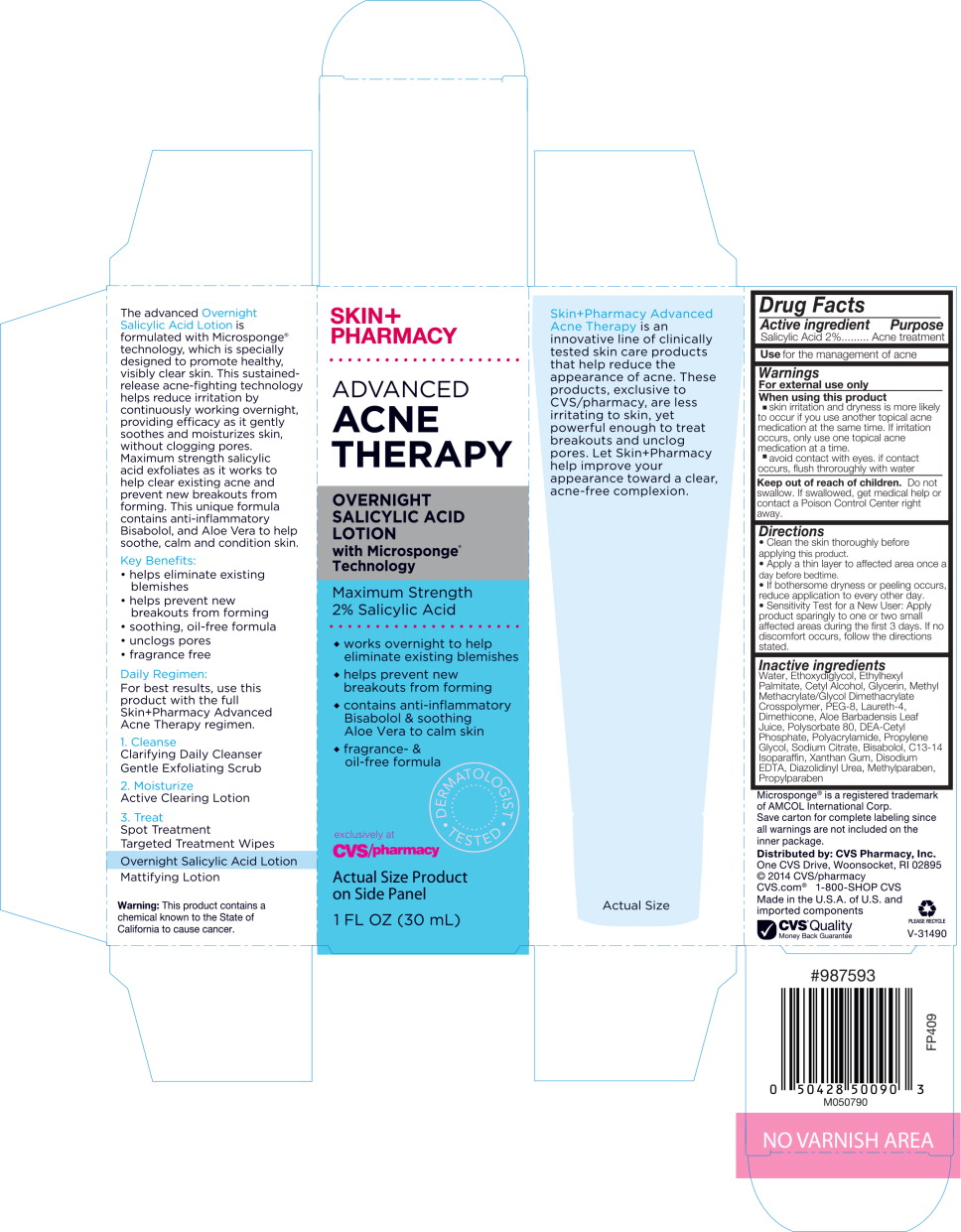
Warnings
For external use only
Directions
- Clean the skin thoroughly before applying this product.
- Apply a thin layer to affected area once a day before bedtime.
- If bothersome dryness or peeling occurs, reduce application to every other day.
- Sensitivity Test for a New User: Apply product sparingly to one or two small affected areas during the first 3 days. If no discomfort occurs, follow the directions stated.
Inactive ingredients
Water, Ethoxydiglycol, Ethylhexyl Palmitate, Cetyl Alcohol, Glycerin, Methyl Methacrylate/Glycol Dimethacrylate Crosspolymer, PEG-8, Laureth-4, Dimethicone, Aloe Barbadensis Leaf Juice, Polysorbate 80, DEA-Cetyl Phosphate, Polyacrylamide, Propylene Glycol, Sodium Citrate, Bisabolol, C13-14 Isoparaffin, Xanthan Gum, Disodium EDTA, Diazolidinyl Urea, Methylparaben, Propylparaben
Microsponge® is a registered trademark of AMCOL International Corp.
Save carton for complete labeling since all warnings are not included on the inner package.
Distributed by: CVS Pharmacy, Inc.
One CVS Drive, Woonsocket, RI 02895
© 2014 CVS/pharmacy
CVS.com® 1-800-SHOP CVS
Made in the U.S.A. of U.S. and foreign components
CVS® Quality
Money Back Guarantee
V-13649
PLEASE RECYCLE
V-31490
#987593
FP409
M050790
Principal Display Panel - Carbon Label
SKIN+
PHARMACY
ADVANCED
ACNE
THERAPY
OVERNIGHT
SALICYLIC
ACID
LOTION
with Microsponge®
Technology
Maximum Strength 2% Salicylic Acid
- works overnight to help eliminate existing blemishes
- helps prevent new breakouts from forming
- contains anti-inflammatory Bisabolol & soothing Ale Vera to calm skin
- fragrance- & oil-free formula
DERMATOLOGIST TESTED
exclusively at
CVS/pharmacy
Actual Size Product
on Side Panel
1 FL OZ (30 mL)
Principal Display Panel - Tube Label
SKIN+PHARMACY
ADVANCED
ACNE
THERAPY
OVERNIGHT
SALICYLIC ACID LOTION
with Microsponge®
Technology
Maximum Strength
2% Salicylic Acid
- works overnight to help eliminate existing blemishes
- helps prevent new breakouts from forming
- contains anti-inflammatory Bisabolol & soothing Aloe Vera to calm skin
- fragrance- & oil-free formula
DERMATOLOGIST TESTED
exclusively at
CVS/pharmacy
1 FL OZ (30 mL)