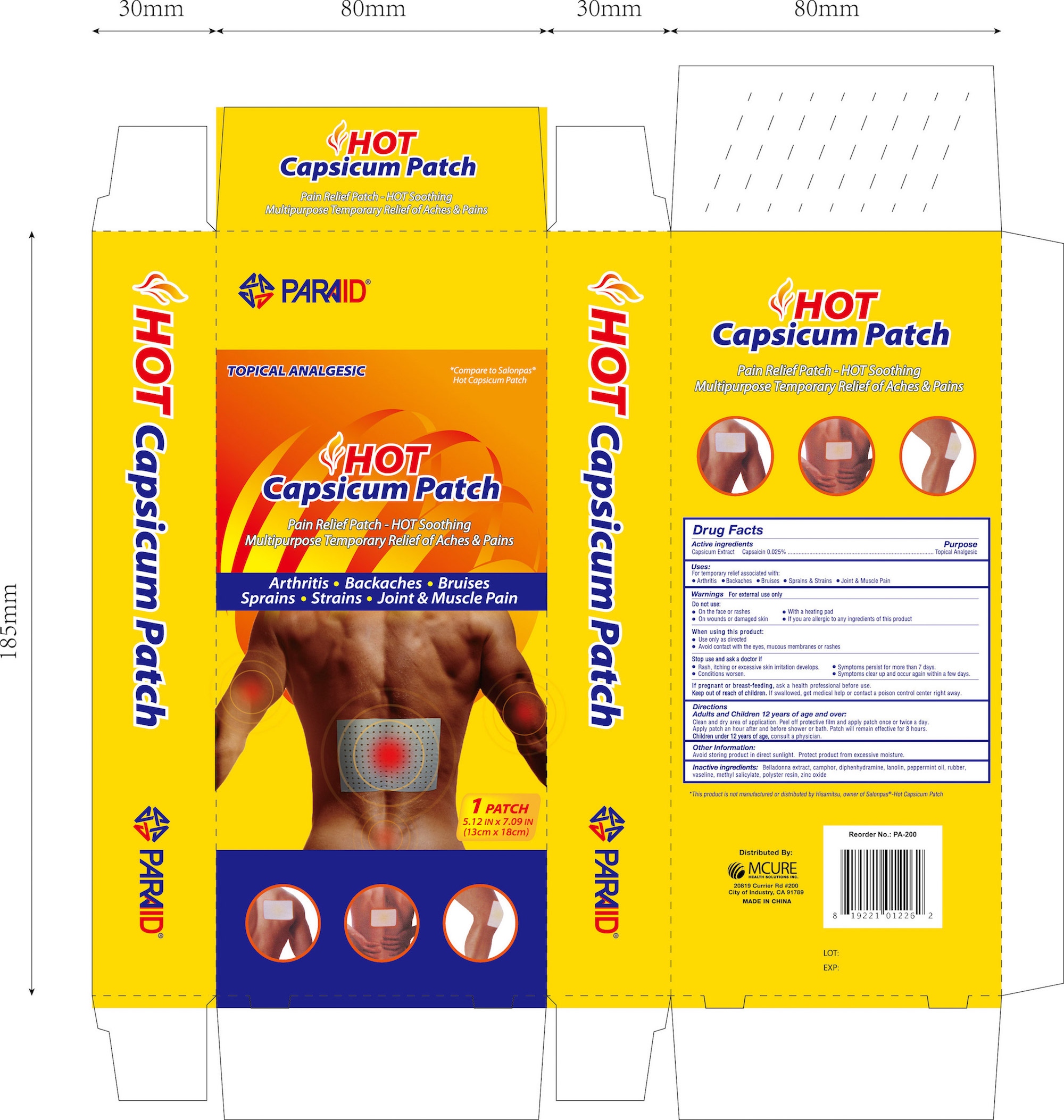
Active ingredients Purpose
Capsicum Extract Capsaicin 0.025%..............................Topical Analgesic
Uses
For temporary relief associated with:
- Arthritis
- Backaches
- Bruises
- Sprains & Strains
- Joint & Muscle Pain
Do not use:
- On the face or rashes
- With a heating pad
- On wounds or damaged skin
- if you are allergic to any ingredients of this product
When using this product:
- Use only as directed
- Avoid contact with the eyes, mucous membranes or rashes
Stop use and ask a doctor if:
- Rash, itching or excessive skin irritation develops
- Conditions worsen
- Symptoms persist for more than 7 days
- Symptoms clear up and occur again within a few days
Keep out of reach of children.
If swallowed, get medical help or contact a Poision Control Center right away.
Directions
Adults and Children 12 years of age and over:
Clean and dry affected area.
Peel off protective film and apply patch once or twice a day.
Apply patch an hour after and before shower or bath. Patch will remain effective for 8 hours.
Children Under 12 Years of Age: Consult a physician.
Inactive ingredients
Belladonna extract, camphor, diphenhydramine, lanolin, peppermint oil, rubber, vaseline, methyl salicylate, polyster resin, zinc oxide