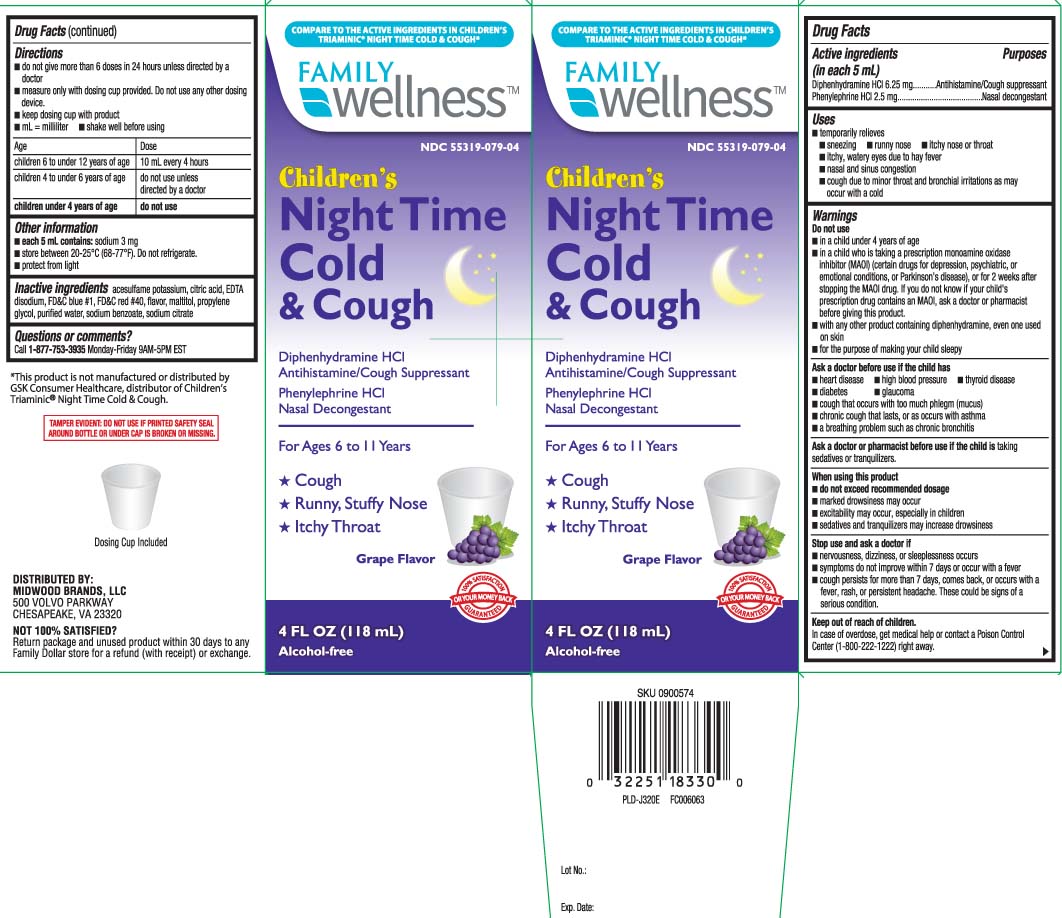
Uses
- temporarily relieves
- sneezing
- runny nose
- itchy nose or throat
- itchy, watery eyes due to hay fever
- nasal and sinus congestion
- cough due to minor throat and bronchial irritations as may occur with a cold
Warnings
Do not use
- in a child under 4 years of age
- in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child's prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product.
- with any other product containing diphenhydramine, even one used on skin
- for the purpose of making your child sleepy
Ask a doctor before use if the child has
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- glaucoma
- cough that occurs with too much phlegm (mucus)
- chronic cough that lasts, or as occurs with asthma
- a breathing problem such as chronic bronchitis
When using this product,
- do not exceed recommended dosage
- marked drowsiness may occur
- excitability may occur, especially in children
- sedatives and tranquilizers may increase drowsiness
Directions
- do not give more than 6 doses in 24 hours unless directed by a doctor.
- mL = milliliter
- measure only with dosing cup provided. Do not use any other dosing device.
- keep dosing cup with product
- shake well before using
| Age | Dose |
| children 6 to under 12 years | 10 mL every 4 hours |
| children 4 to under 6 years | do not use unless directed by a doctor |
| children under 4 years | do not use |
Other Information
- each 5 mL contains: sodium 3 mg
- store between 20-25ºC (68-77ºF). do not refrigerate
- protect from light
Inactive ingredients
acesulfame potassium, citric acid, EDTA disodium, FD&C blue #1, FD&C red #40, flavor, maltitol, propylene glycol, purified water, sodium benzoate, sodium citrate
Principal Display Panel
COMPARE TO ACTIVE INGREDIENTS OF CHILDREN'S TRIAMINIC® NIGHTTIME COLD & COUGH*
Children's
Cold & Cough Nighttime
Diphenhydramine HCL Antihistamine/Cough Suppressant
Phenylephrine HCL Nasal Decongestant
For Ages 6 to 11 Years
- Cough
- Runny, Stuffy Nose
- Itchy Throat
Alcohol-Free
FL OZ (mL)
GRAPE FLAVOR
Dosing Cup Included
*This product is not manufactured or distributed by GSK, Consumer Healthcare, distributor of Children's Triaminic® NightTime Cold & Cough.
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF PRINTED SAFETY SEAL AROUND BOTTLE OR UNDER CAP IS BROKEN OR MISSING.
DISTRIBUTED BY:
MIDWOOD BRANDS, LLC
500 VOLVO PARKWAY
CHESAPEAKE, VA 23320