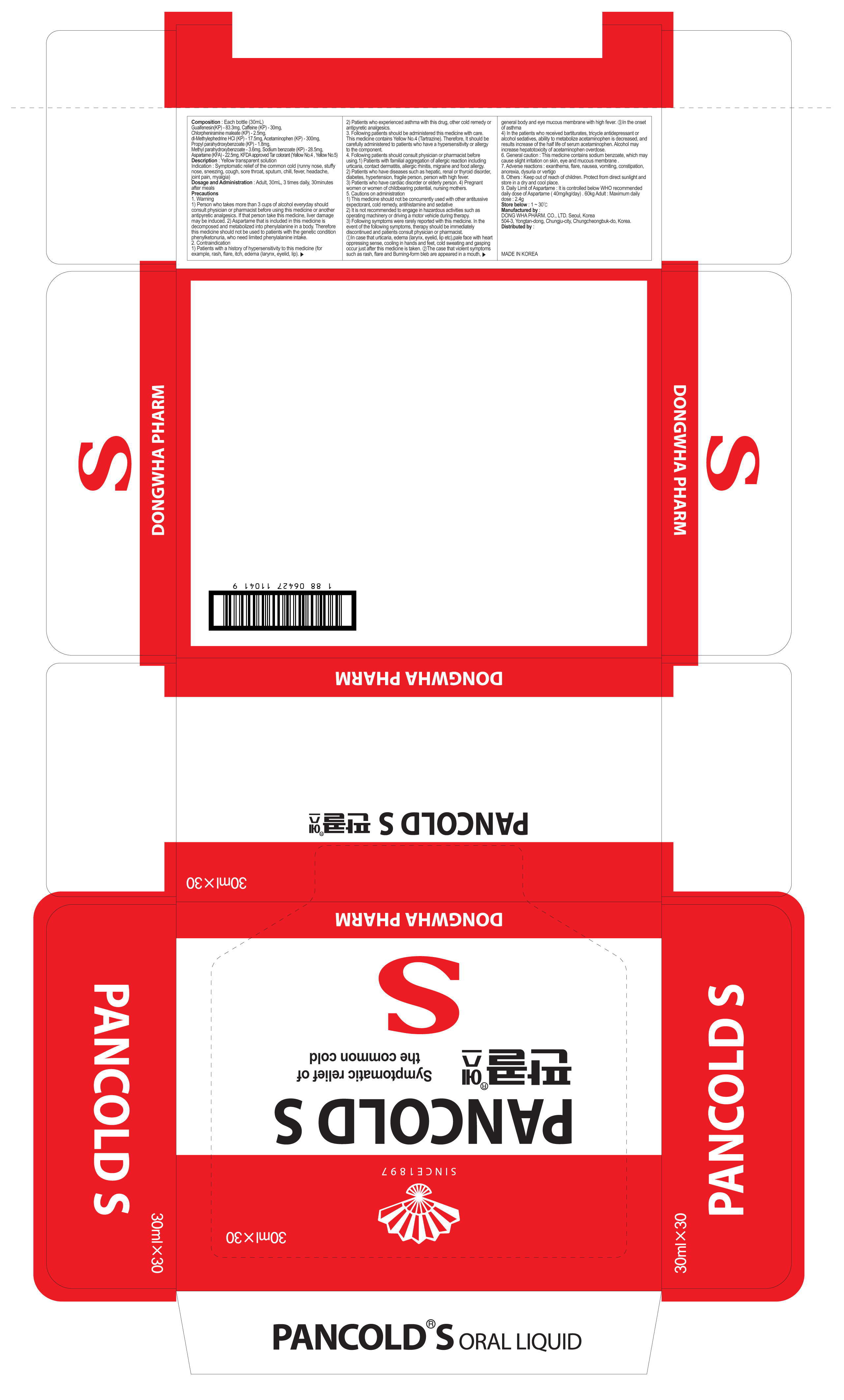
alcohol, citric acid, edentate sodium, high fructose corn syrup, lemon essence, methylparaben, monosodium glutamate, orange essence, propylene glycol, propylparaben, sodium benzoate, sodium chloride, water, tartrazine
Temporarily relieves these minor symptoms due to a cold or the flu:
▪aches ▪pain ▪headache ▪sore throat ▪muscular aches ▪fever
▪rummy nose ▪sneezing ▪itching of the nose and throat
▪nasal congestion ▪sinus congestion and pressure
▪cough due to minor throat and bronchial irritation
▪aches ▪pain ▪headache ▪sore throat ▪muscular aches ▪fever
▪rummy nose ▪sneezing ▪itching of the nose and throat
▪nasal congestion ▪sinus congestion and pressure
▪cough due to minor throat and bronchial irritation
Adults and children 12 years and older: Take 1 bottle every 4 hours, while symptoms persist not to exceed 6 bottles in 24 hours, or as directed by a doctor.