Rx Only
DESCRIPTION
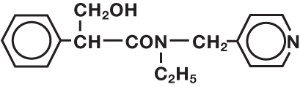
Tropicamide Ophthalmic Solution, USP, 0.5% is an anticholinergic prepared as a sterile topical ophthalmic solution. The active ingredient is represented by the chemical structure:
Established name: Tropicamide ophthalmic solution
Chemical name: Benzeneacetamide, N-ethyl-α-(hydroxymethyl)-N-(4-pyridinylmethyl)-.
Each mL contains: Active: tropicamide 0.5%. Preservative: benzalkonium chloride 0.01%. Inactives: sodium chloride, edetate disodium, hydrochloric acid and/or sodium hydroxide (to adjust pH), purified water. pH 4.0 - 5.8.
CLINICAL PHARMACOLOGY
This anticholinergic preparation blocks the responses of the sphincter muscle of the iris and the ciliary muscle to cholinergic stimulation, dilating the pupil (mydriasis). The stronger preparation (1%) also paralyzes accommodation. This preparation acts in 15 to 30 minutes, and the duration of activity is approximately 3 to 8 hours. Complete recovery from mydriasis in some individuals may require 24 hours. The weaker strength may be useful in producing mydriasis with only slight cycloplegia. Heavily pigmented irides may require more doses than lightly pigmented irides.
CONTRAINDICATIONS
Contraindicated in persons showing hypersensitivity to any component of this preparation.
WARNINGS
For topical ophthalmic use only. Not for injection.
This preparation may cause CNS disturbances which may be dangerous in pediatric patients. The possibility of psychotic reactions and behavioral disturbances due to hypersensitivity to anticholinergic drugs should be considered.
Mydriatics may produce a transient elevation of intraocular pressure.
Remove contact lenses before using.
PRECAUTIONS
General
The lacrimal sac should be compressed by digital pressure for two to three minutes after instillation to reduce excessive systemic absorption.
Information for Patients
Do not touch dropper tip to any surface, as this may contaminate the solution. Patient should be advised not to drive or engage in potentially hazardous activities while pupils are dilated. Patient may experience sensitivity to light and should protect eyes in bright illumination during dilation. Parents should be warned not to get this preparation in their child's mouth and to wash their own hands and the child's hands following administration.
Drug Interactions
Tropicamide may interfere with the antihypertensive action of carbachol, pilocarpine, or ophthalmic cholinesterase inhibitors.
Carcinogenesis, Mutagenesis, Impairment of Fertility
There have been no long-term studies done using tropicamide in animals to evaluate carcinogenic potential.
Pregnancy
Animal reproduction studies have not been conducted with tropicamide. It is also not known whether tropicamide can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Tropicamide should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when tropicamide is administered to a nursing woman.
Pediatric Use
Tropicamide may rarely cause CNS disturbances which may be dangerous in pediatric patients. Psychotic reactions, behavioral disturbances, and vasomotor or cardiorespiratory collapse in children have been reported with the use of anticholinergic drugs [see Warnings]. Keep this and all medications out of the reach of children.
ADVERSE REACTIONS
Ocular
Transient stinging, blurred vision, photophobia and superficial punctate keratitis have been reported with the use of tropicamide. Increased intraocular pressure has been reported following the use of mydriatics.
Non-Ocular
Dryness of the mouth, tachycardia, headache, allergic reactions, nausea, vomiting, pallor, central nervous system disturbances and muscle rigidity have been reported with the use of tropicamide. Psychotic reactions, behavioral disturbances, and vasomotor or cardio-respiratory collapse in children have been reported with the use of anticholinergic drugs.
To report SUSPECTED ADVERSE REACTIONS, contact Sandoz Inc. at 1-800-525-8747 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DOSAGE AND ADMINISTRATION
For examination of fundus, instill one or two drops of 0.5% solution 15 or 20 minutes prior to examination. Individuals with heavily pigmented irides may require higher strength or more doses. Mydriasis will reverse spontaneously with time, typically in 4 to 8 hours. However, in some cases, complete recovery may take up to 24 hours.
HOW SUPPLIED
Tropicamide ophthalmic solution, USP, 0.5% is supplied in an LDPE plastic bottle with a dropper tip as:
- NDC 61314-354-01 15mL
STORAGE
Store at 8° to 25°C (46° to 77°F). Do not refrigerate or store at high temperatures. Keep container tightly closed.
After opening, this product can be used until the expiration date on the bottle.