Warnings
For external use only.
Principal Display Panel - 0.13 g/100 g Case Label
NDC 68599-5806-3
268
McKESSON
Benzalkonium Chloride Towelettes
ANTISEPTIC | GERMICIDAL | STERILE
100
PER BOX
10
BOXES PER CASE
DO NOT REUSE
Store at 59–86°F (15–30°C). Contents STERILE in unopened, undamaged inner package. Not made with natural rubber latex.
Distributed By McKesson Medical-Surgical Inc.
Richmond, VA 23233
Rev. 00 04/16
Made in China
STERILE R
MFR # 268
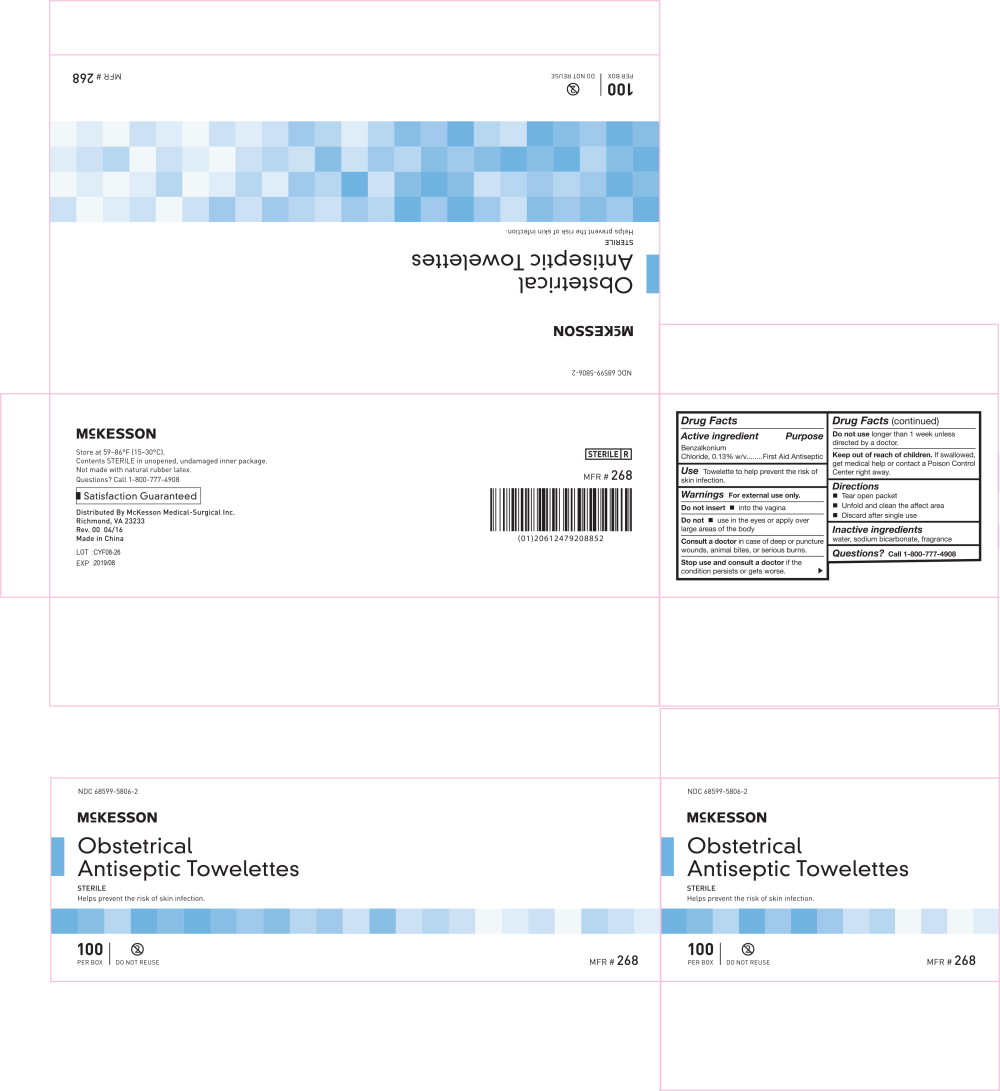
Principal Display Panel - 0.13 g/100 g Box Label
NDC 68599-5806-2
McKESSON
Benzalkonium
Chloride Towelettes
ANTISEPTIC | GERMICIDAL | STERILE
Helps prevent the risk of skin infection.
100
PER BOX
DO NOT REUSE
MFR # 268
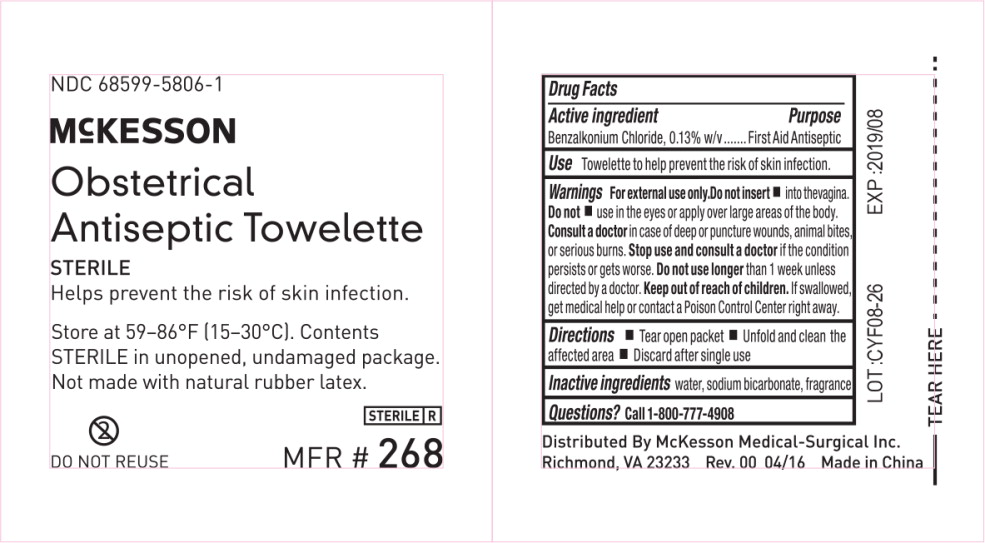
Principal Display Panel - 0.13 g/100 g Packet Label
NDC 68599-5806-1
McKESSON
Benzalkonium
Chloride Towelettes
ANTISEPTIC | GERMICIDAL | STERILE
Helps prevent the risk of skin infection.
Store at 59–86°F (15–30°C). Contents
STERILE in unopened, undamaged package.
Not made with natural rubber latex.
DO NOT REUSE
STERILE R
MFR # 268