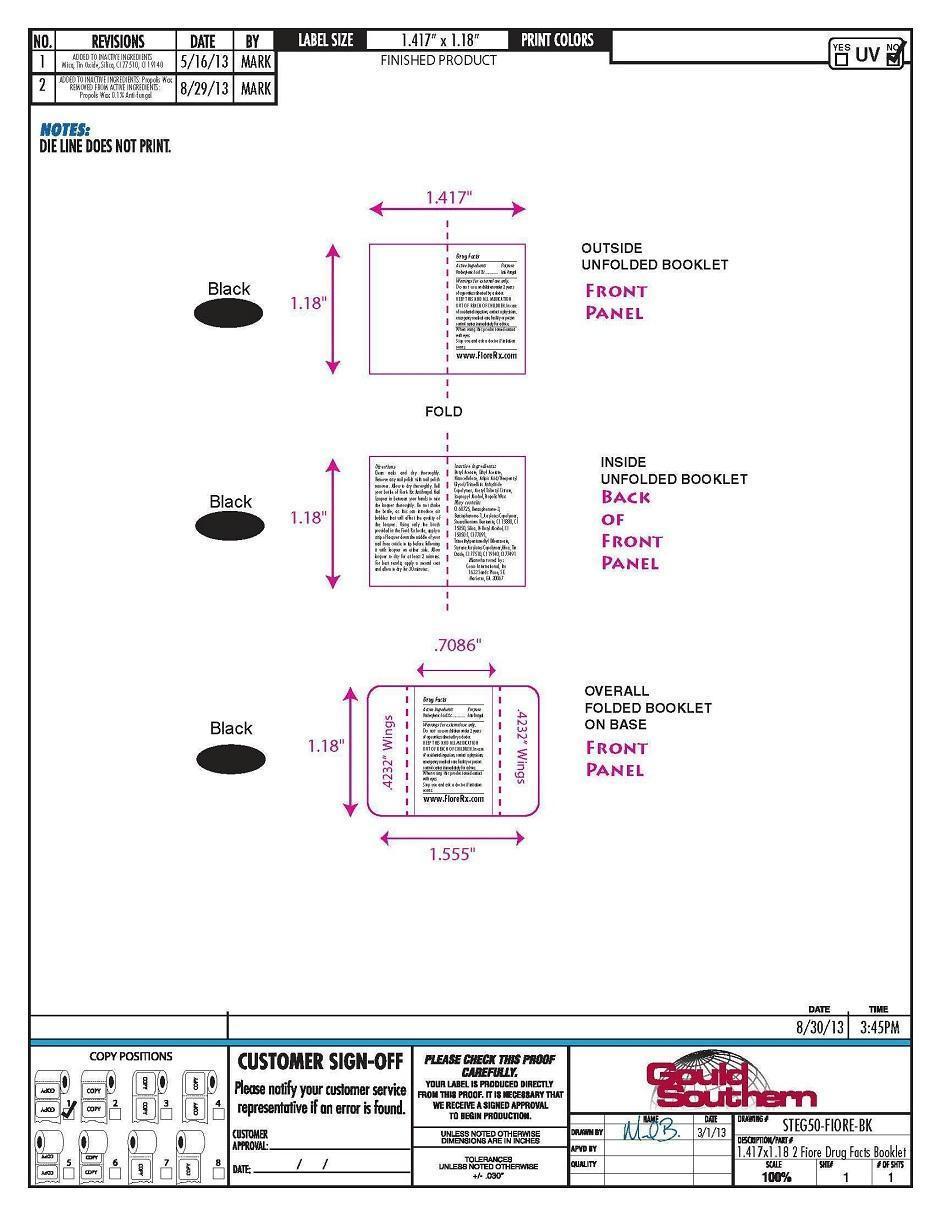
Do not use on children under 2 years of age unless directed by a doctor.
KEEP THIS AND ALL MEDICATION OUT OF REACH OF CHILDREN.
In case of accidental ingestion, contact a physician, emergency medical care facility or poison control center immediately for advice.
Directions
Clean nails and dry thoroughly.
Remove any nail polish with nail
polish remover. Allow to dry
thoroughly. Roll your bottle of Fioré
Rx Antifungal Nail Lacquer in between
your hands to mix the lacquer
thoroughly. Do not shake the bottle,
as this can introduce air bubbles that
will affect the quality of the lacquer.
Using only the brush provided in the
Fioré Rx bottle, apply a strip of
lacquer down the middle of your nail
from cuticle to tip before following it
with lacquer on either side. Allow
lacquer to dry for at least 2 minutes.
For best results, apply a second coat
and allow to dry for 30 minutes.