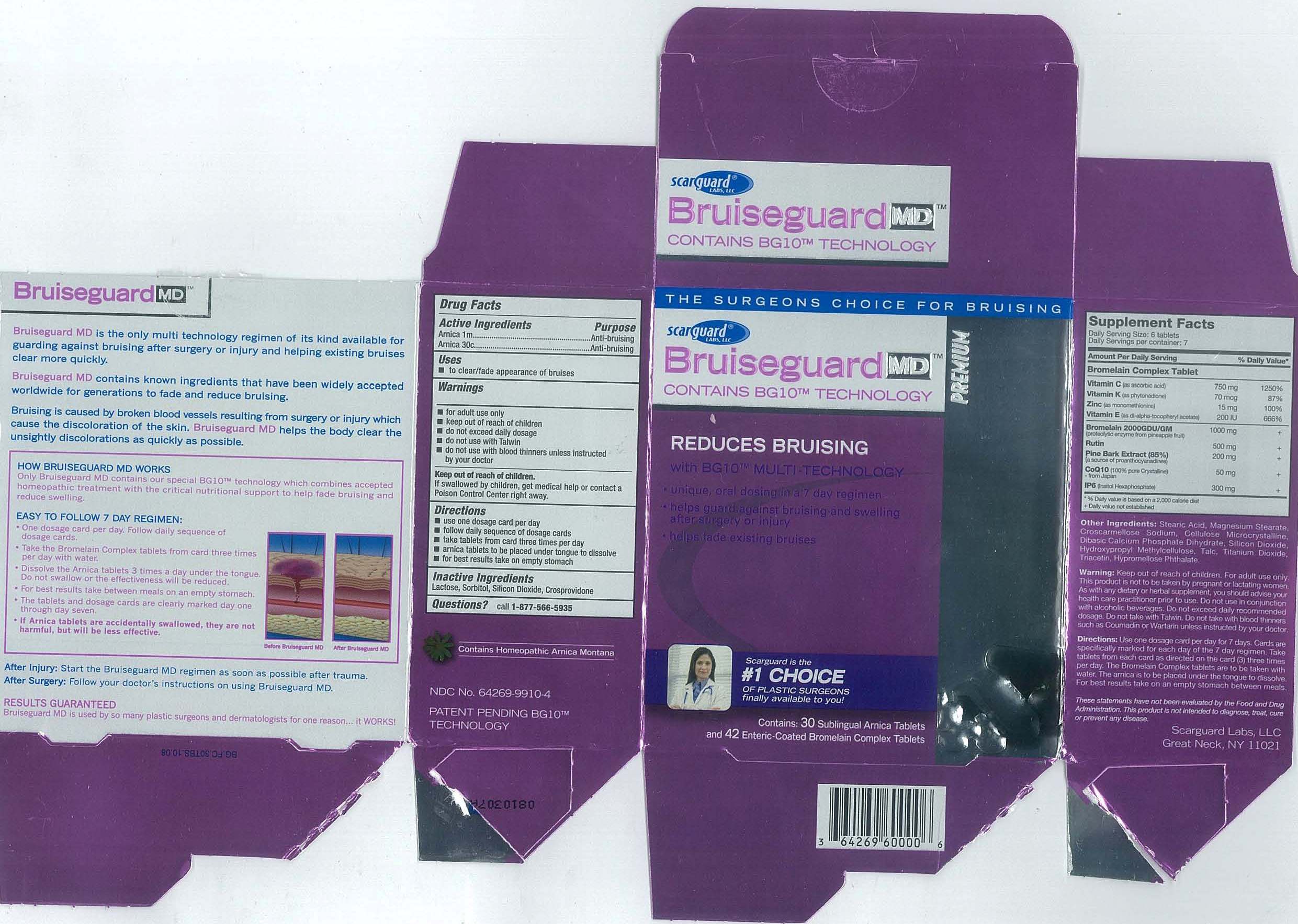
BRUISEGUARD MD- arnica montana
Scarguard Labs, LLC
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Active Ingredients
Arnica 1m
Arnica 30c
Purpose
Anti-bruising
Anti-bruising
Uses
to clear/fade appearance of bruises
Warnings
- for adult use only
- keep out of reach of children
- do not exceed daily dosage
- do not use with Talwin
- do not use with blood thinners unless instructed by your doctor
Keep out of reach of children
If swallowed by children, get medical help or contact a Poison Control Center right away.
Directions
- use one dosage card per day
- follow daily sequence of dosage cards
- take tablets from card three times per day
- arnica tablets to be placed under tongue to dissolve
- for best results take on empty stomach
Inactive ingredients
Lactose, Sorbitol, Silicone Dioxide, Crospovidone
Bruiseguard MD carton