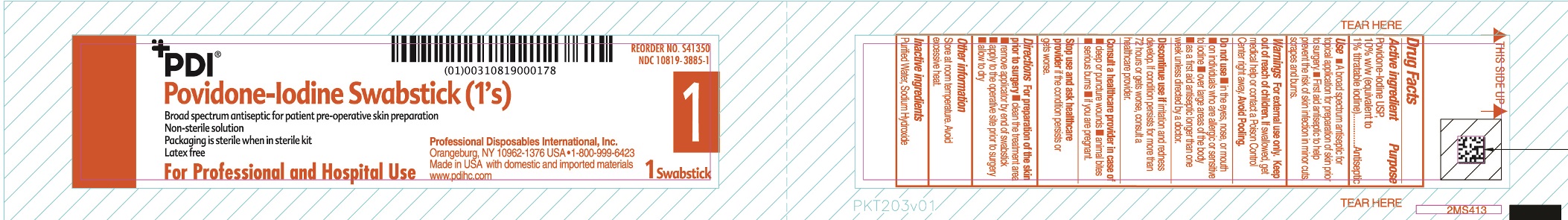
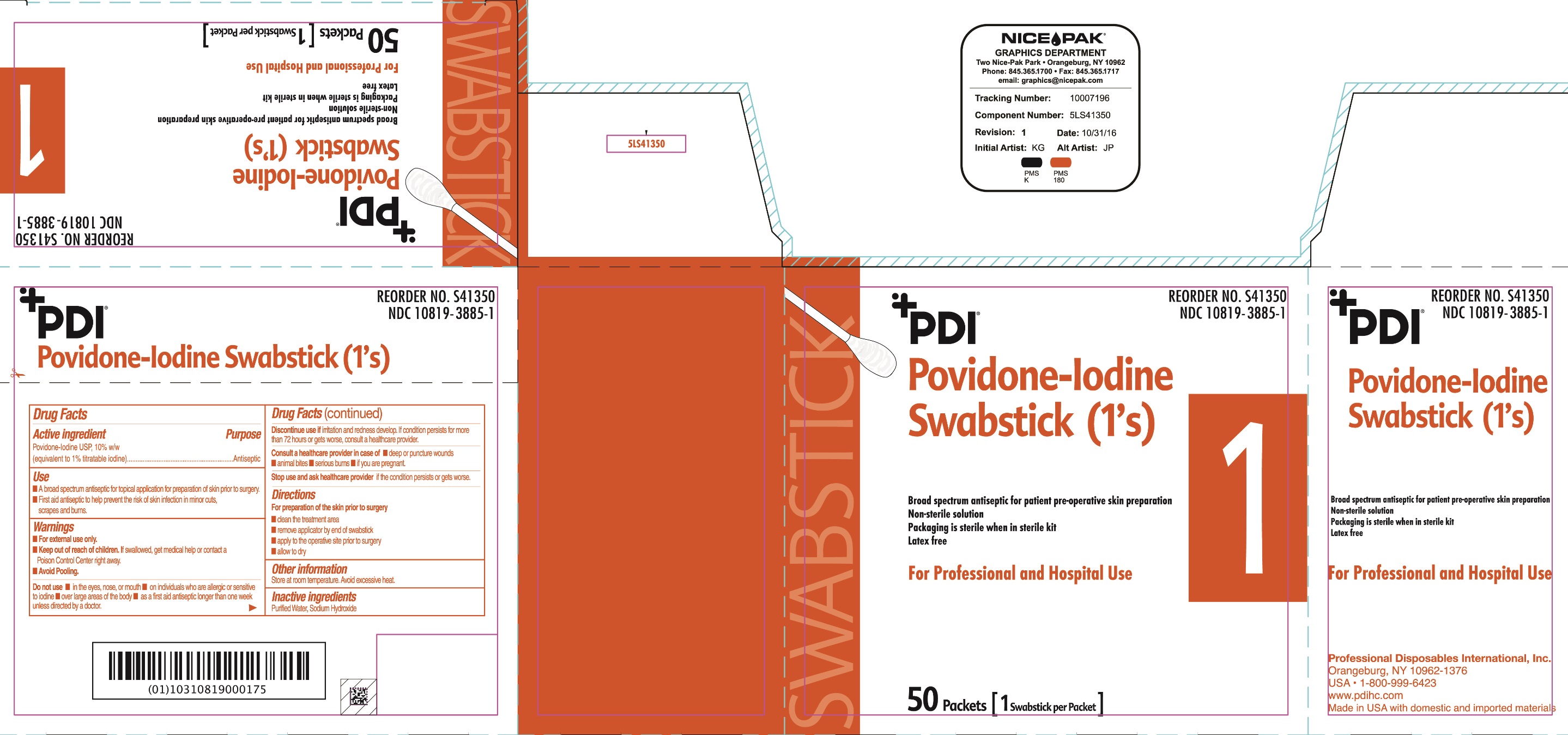
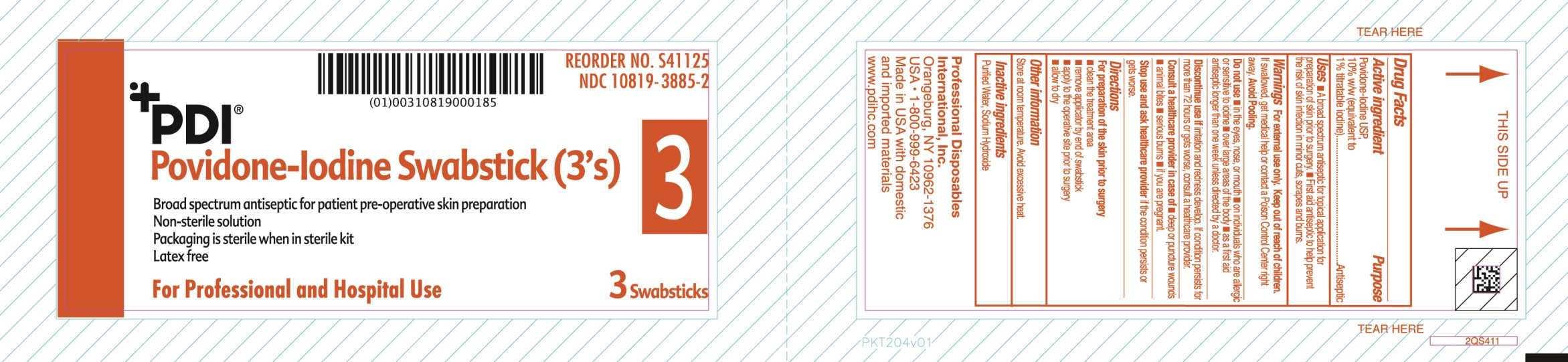
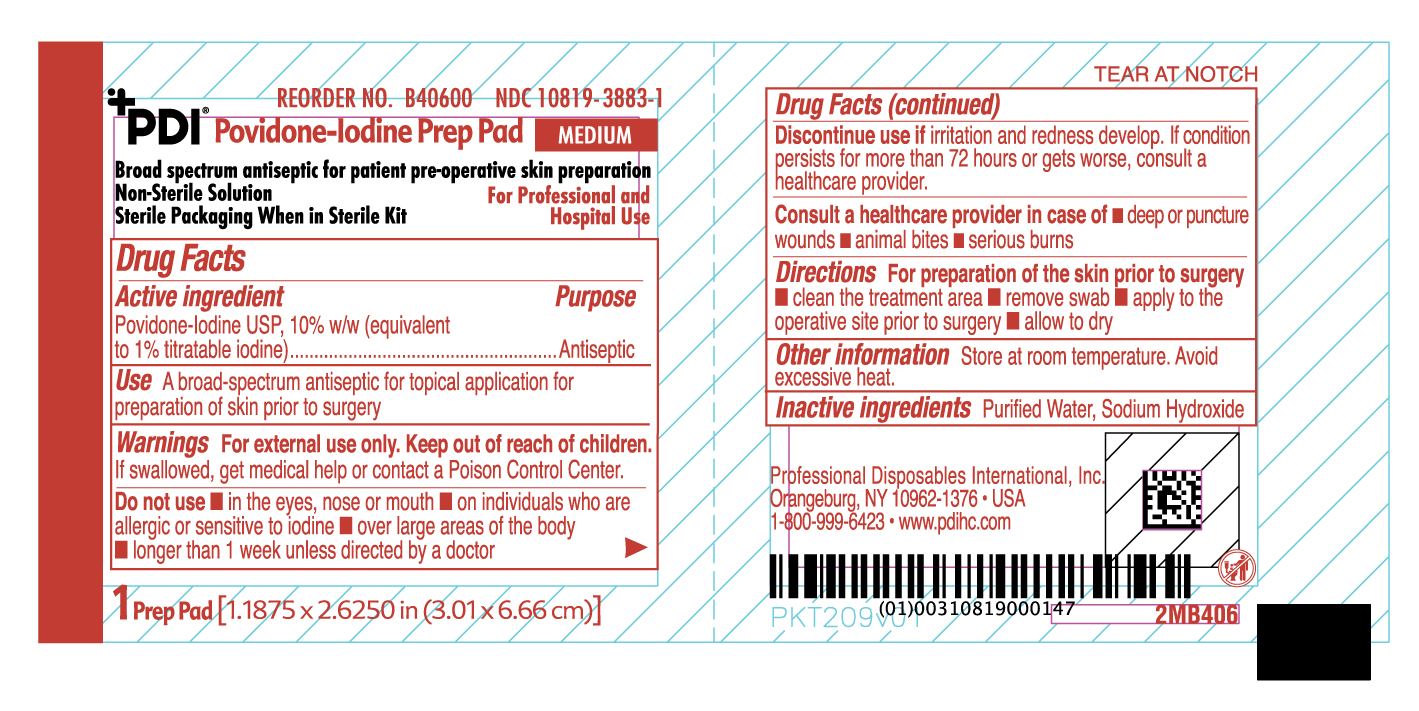
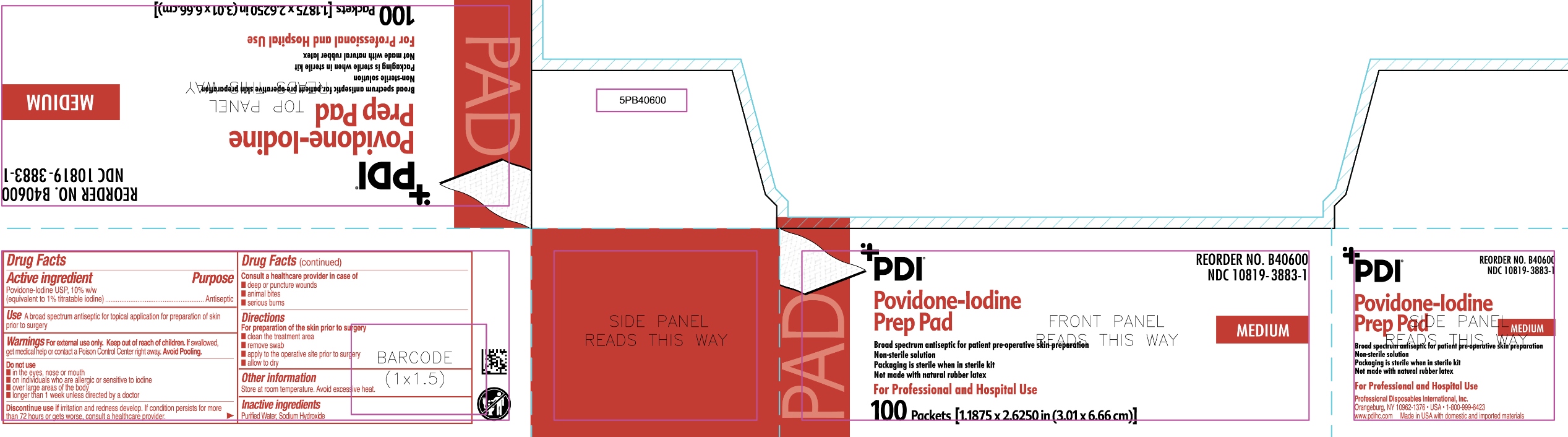
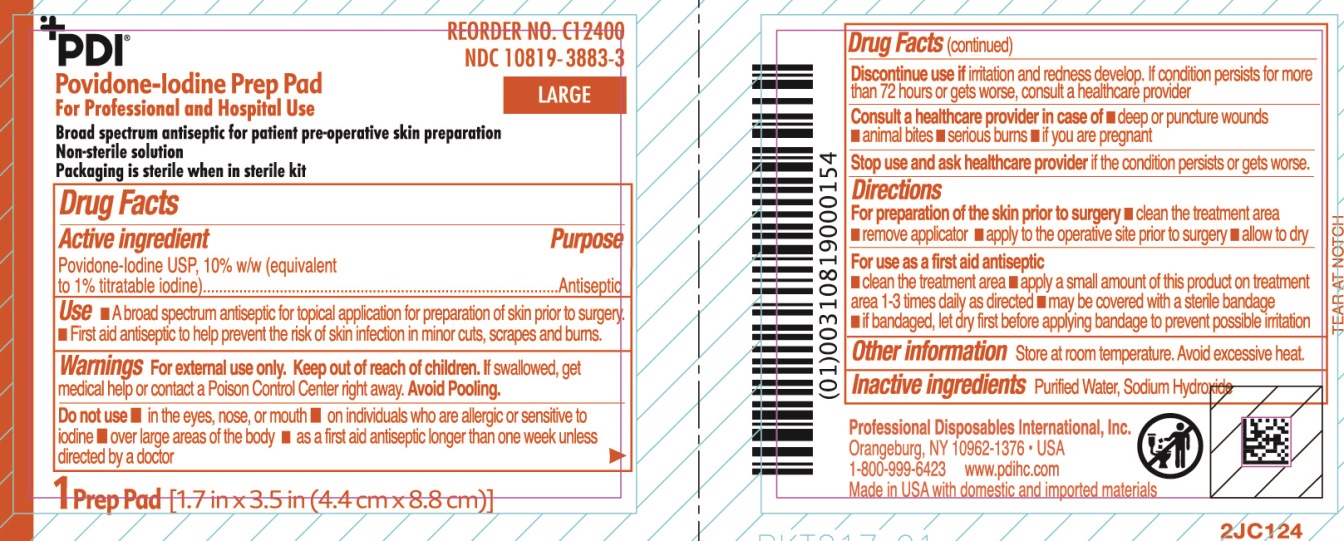
Uses
- A broad spectrum antiseptic for topical application for preparation of the skin prior to surgery.
Warnings
For external use only
If swallowed, get medical help or contact a Poison Control Center.
Discontinue use if irritation and redness develop. If condition persist for more than 72 hours or gets worse, consult a healthcare provider
Directions
For preparation of the skin prior to surgery
- clean the treatment area
- remove swab
- apply to the operative site prior to surgery
- allow to dry
Do not use
Do not use
- in the eyes, nose or mouth
- on individuals who are allergic or sensitive to iodine
- over large areas of the body
- longer than one week unless directed by a doctor