HAN-I-FOAM
- alcohol liquid
Momar Incorporated
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
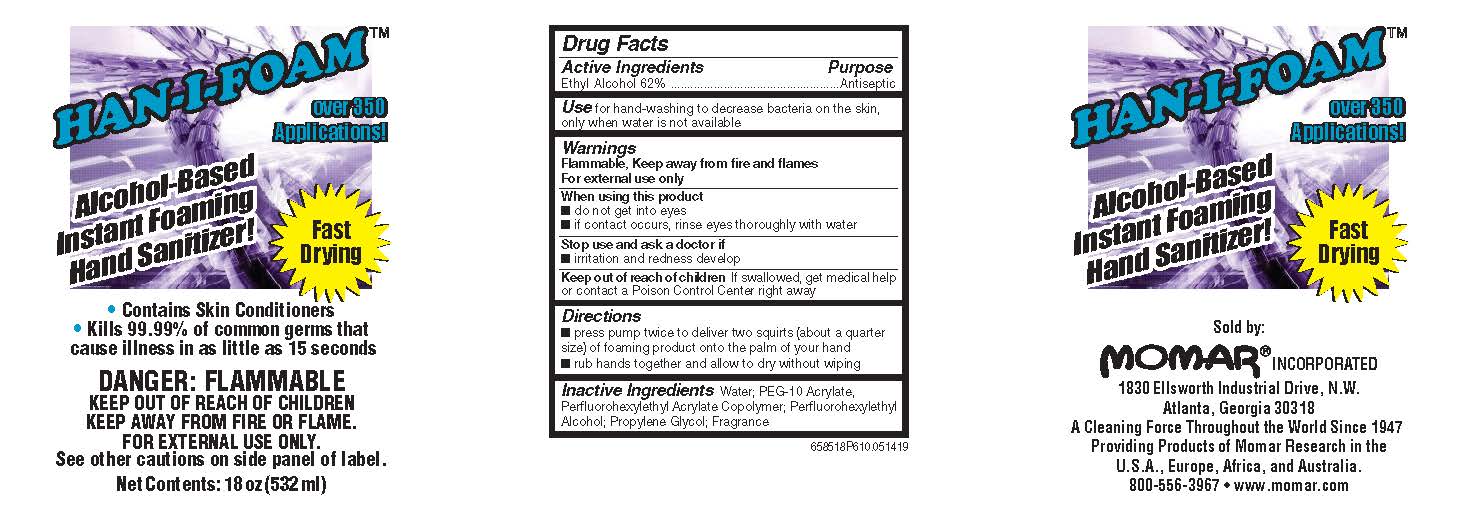
Drug Facts Box OTC-Active Ingredient Section
Ethyl Alcohol 62%
Drug Facts Box OTC-Purpose Section
Antiseptic
Drug Facts Box OTC-Indications & Usage Section
for hand-washing to decrease bacteria on the skin, only when water is not available
Drug Facts Box OTC-Warnings Section
FLAMMABLE, keep away from fire and flames
For external use only
Drug Facts Box OTC-When Using Section
do not get into eyes
if contact occurs, rinse eyes thoroughly with water
Drug Facts Box OTC-Stop Use Section
irritation and redness develop
Drug Facts Box OTC-Keep Out of Reach of Children Section
if swallowed, get medical help or contact a Poison Control Center right away
Drug Facts Box OTC-Dosage & Administration Section
press pump twice to deliver two squirts (about a quarter size) of foaming product onto the palm of your hand
rub hands together and allow to dry without wiping
Drug Facts Box OTC-Inactive Ingredient Section
water, PEG-10 acrylate, perfluorohexylethyl acrylate copolymer, propylene glycol, fragrance
Han-I-Foam 6585

Han-I-Foam 6585
