Warnings
For external use only.
Flammable. Keep away from sparks, heat and fire.
Consult a doctor before use if you have - deep or puncture wounds - animal bites - serious burns
Directions
- adults and children 2 years of age and older: clean the affected area
- apply a small amount of this product to the area 1 to 3 times daily _ may be covered with a sterile bandage. If bandaged, let dry first
- children under 2 years of age: ask a doctor
Product Labeling
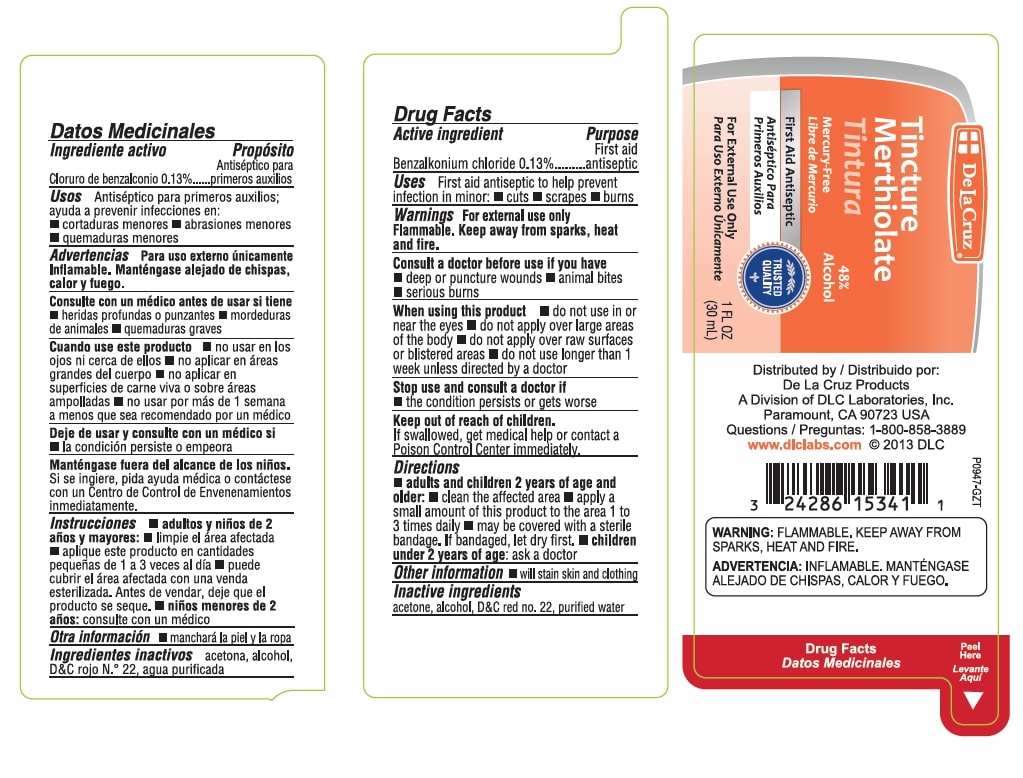
Tincture Merthiolate
48% Alcohol
Mercury Free
First Aid Antiseptic
For External Use Only
1 FL OZ (30 mL)
Distributed by
De La Cruz Products
A Division of DLC Laboratories, Inc.
Paramount, CA 90723 USA
Questions: 1-800-858-3889
www.dlclabs.com
Warnings: FLAMMABLE, KEEP AWAY FROM SPARKS, HEAT AND FIRE.