Uses
- temporarily relieves minor aches and pains due to:
- headache
- the common cold
- backache
- minor pain of arthritis
- toothache
- muscular aches
- premenstrual and menstrual cramps
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- blisters
- rash
- skin reddening
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Directions
-
do not take more than directed
- adults and children 12 years and over
- take 2 gelcaps every 6 hours while symptoms last
- do not take more than 6 gelcaps in 24 hours, unless directed by a doctor
- do not take for more than 10 days unless directed by a doctor
- children under 12 years: ask a doctor
Other information
- store at 25ºC (77ºF); excursions permitted between 15°-30°C (59°-86°F)
- use by expiration date on package
- avoid high humidity
Inactive ingredients
croscarmellose sodium, D&C red #33, FD&C blue #1, FD&C red #40, gelatin, hydroxypropyl cellulose, hypromellose, iron oxide black, iron oxide red, iron oxide yellow, polyethylene glycol, povidone, pregelatinized starch, propylene glycol, shellac glaze, stearic acid, titanium dioxide
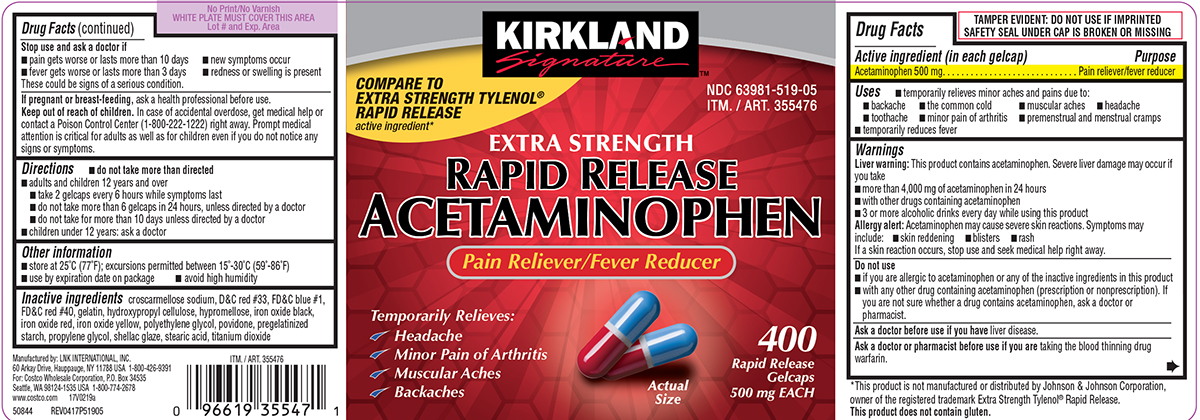
Primary Display Panel
KIRKLAND
Signature™
COMPARE TO
EXTRA STRENGTH TYLENOL®
RAPID RELEASE active ingredient*
NDC 63981-519-05
ITM. / ART. 355476
EXTRA STRENGTH
RAPID RELEASE
ACETAMINOPHEN
Pain Reliever/Fever Reducer
Temporarily Relieves:
✓ Headache
✓ Minor Pain of Arthritis
✓ Muscular Aches
✓ Backaches
Actual
Size
400
Rapid Release
Gelcaps
500 mg EACH
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
*This product is not manufactured or distributed by Johnson & Johnson Corporation,
owner of the registered trademark Extra Strength Tylenol® Rapid Release.
This product does not contain gluten.
Manufactured by: LNK INTERNATIONAL, INC.
60 Arkay Drive, Hauppauge, NY 11788 USA 1-800-426-9391
For: Costco Wholesale Corporation, P.O. Box 34535
Seattle, WA 98124-1535 USA 1-800-774-2678
www.costco.com 17V0219a
50844 REV0417P51905
44-519