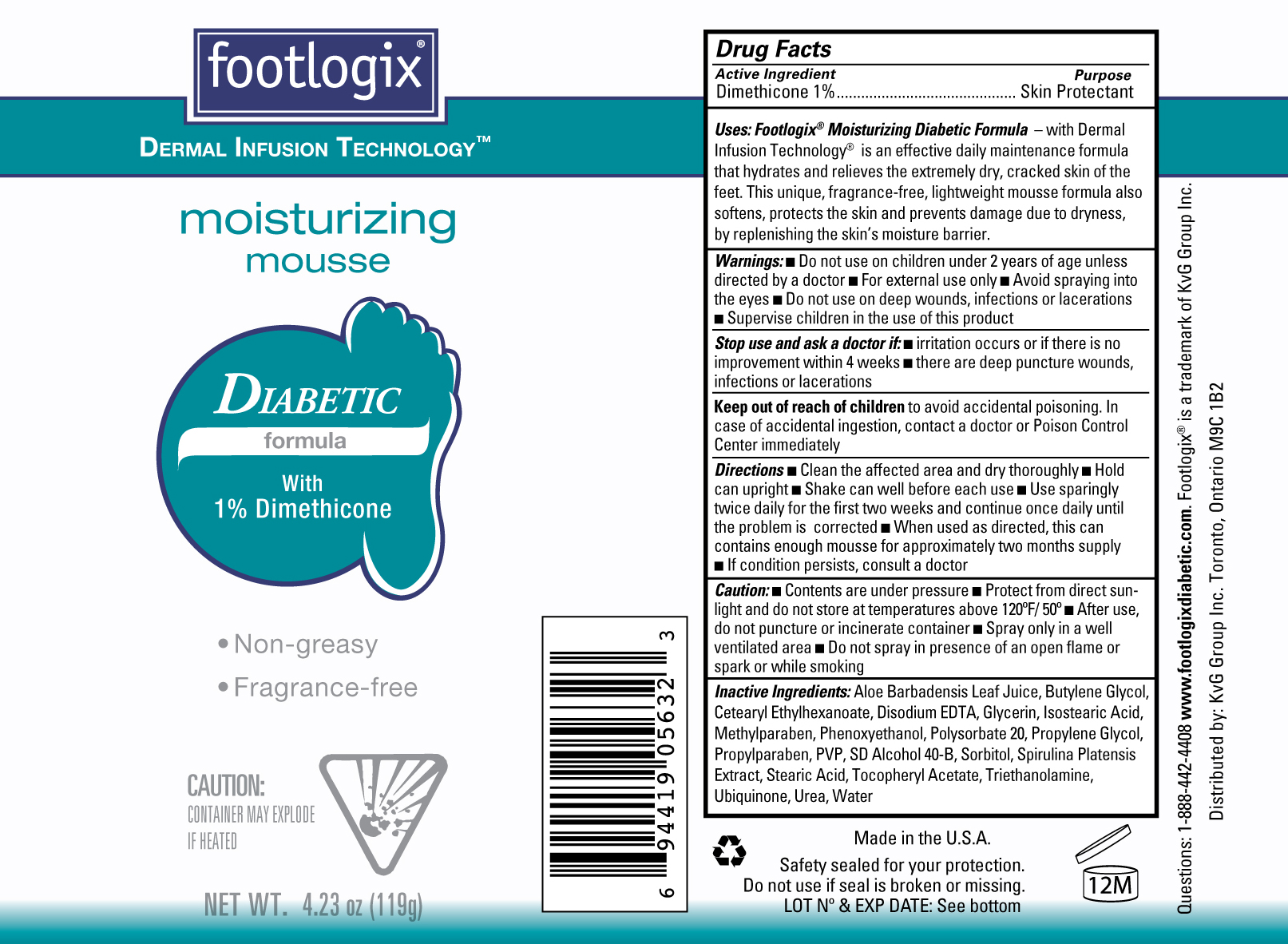
Uses: Footlogix Moisturizing Diabetic Formula- with Dermal Infusion Technology is an effective daily maintenance formula that hydrates and relieves the extremely dry, cracked skin of the feet. This unique fragrance-free, lightweight mousse formula also softens, protects the skni and prevents damage due to dryness by replenishing the skin's moisture barrier.
Warnings ■ Do not use on children 2 years of age unless directed by a doctor ■ For external use only ■ Avoid spraying into the eyes ■ Do not use on deep wounds, infections or lacerations ■ Supervise children in the use of this product
Stop use and ask a doctor if: ■ irritation occurs or if there is no improvement within 4 weeks ■ there are deep puncture wounds, infections or lacerations
Keep out of reach of children to avoid accidental poisoning. In case of accidental ingestion, contact a doctor or Poison Control Center immediately
Caution: ■ Contents are under pressure ■ Protect from direct sunlight and do not store at temperatures above 120F/50C ■ After use, do not puncture or incinerate container ■ Spray only in a well ventilated area ■ Do not spray in presence of open flame or spark or while smoking
Inactive Ingredients Aloe Barbadensis Leaf Juice, Butylene Glycol, Cetearyl Ethylhexanoate, Disodium EDTA, Glycerin, Isostearic Acid, Methylparaben, Phenoxyethanol, Polysorbate 20, Propylene Glycol, Propylparaben, PVP, SD Alcohol 40-B, Sorbitol, Spirulina Platensis Extract, Stearic Acid, Tocopheryl Acetate, Triethanolamine, Ubiquinone, Urea, Water.