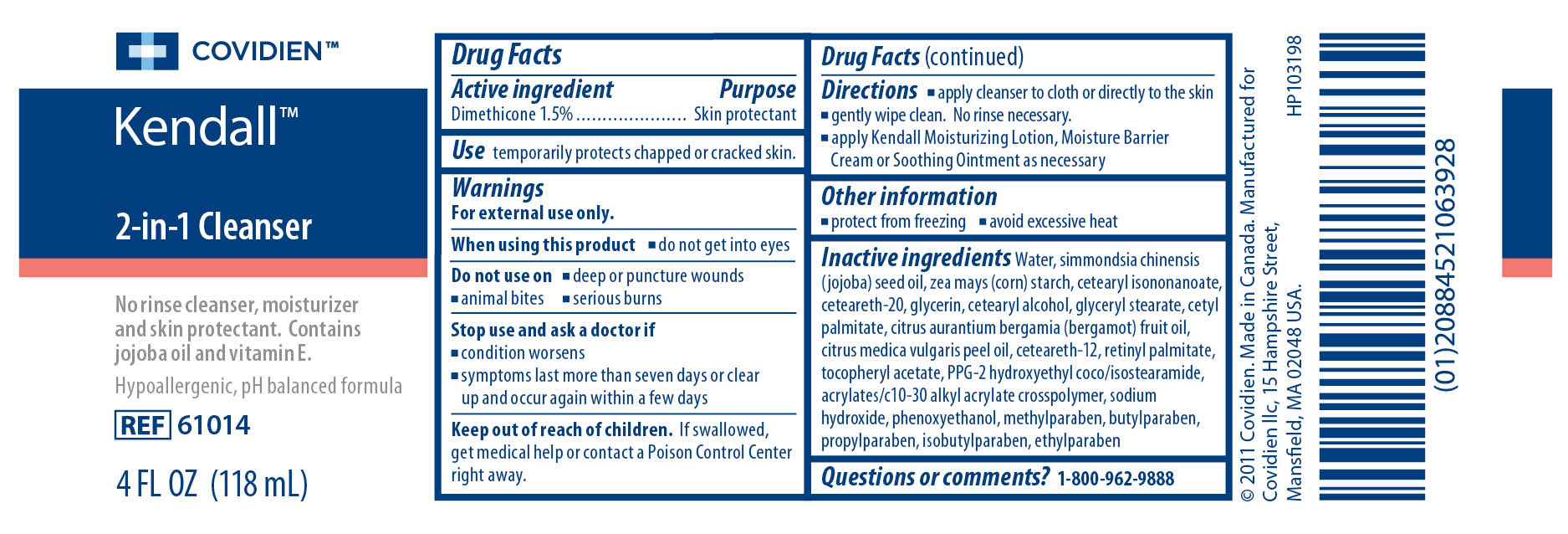
Warnings
For external use only.When using this product
- do not get into eyes
- deep or puncture wounds
- animal bites
- serious burns
- condition worsens
- symptoms last more than seven days or clear up and occur again within a few days
Directions
- apply cleanser to cloth or directly to the skin
- gently wipe clean. No rinse necessary.
- apply Kendall Moisturizing Lotion, Moisture Barrier Cream or Soothing Ointment as necessary
Inactive ingredients
Water, simmondsia chinensis (jojoba) seed oil, zea mays (corn) starch, cetearyl isononanoate, ceteareth-20, glycerin, cetearyl alcohol, glyceryl stearate, cetyl palmitate, cirtus aurantium bergamia (bergamot) fruit oil, citrus medica vulgaris peel oil, ceteareth-12, retinyl palmitate, tocopheryl acetate, PPG-2 hydroxyethyl coco/isostearamide, acrylates/c10-30 alkyl acrylate crosspolymer, sodium hydroxide, phenoxyethanol, methylparaben, butylparaben, propylparaben, isobutylparaben, ethylparaben