GLIZIGEN SKIN PROTECTANT- glycerin spray
Catalysis, SL
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Glycerin 0.5%................Skin Protectant
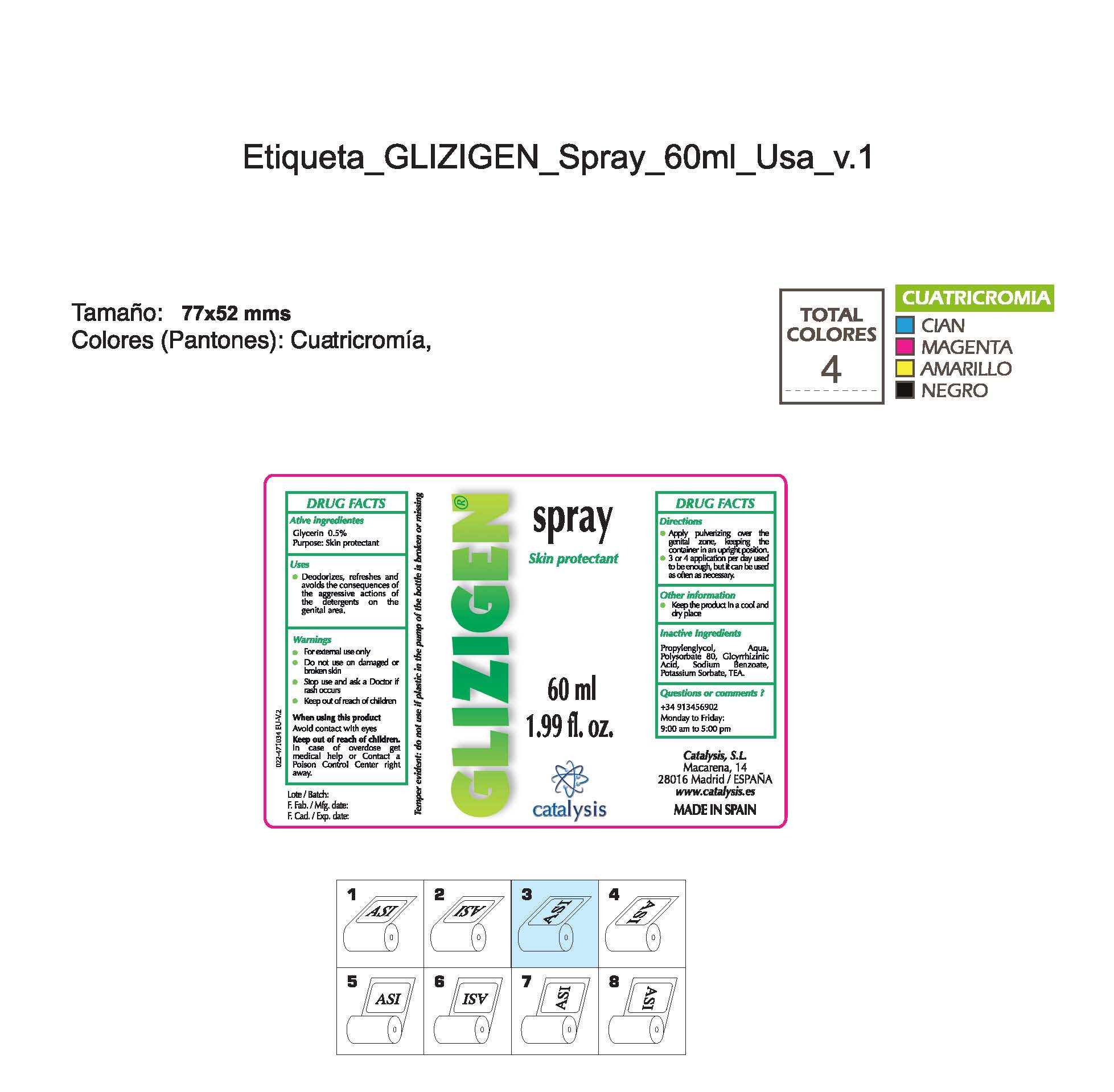
- Stop use and ask a doctor if rash occurs
- Children under 6 months: ask a doctor
- Do not use on damaged or broken skin.
- Keep out of reach of children
- For external use only.
- Do not use on damaged or broken skin.
- When using this product keep our of the eyes. Rinse with water to remove.
- Stop use and ask a doctor if rash occurs.
- Keep out of reach of children
- Children under 6 months: as a doctor
- + 34 913456902 M-F 9:00 am to 5:00 pm
- deodorizes, refreshes and avoids the consequences of the agressive actions of the detergents in the genital areas
- apply pulverizing over the genital zone keeping the container in an upright position
- 3 or 4 applications per day used to be anough, but it can be used as often as necessary
- apply pulverizing over the genital zone keeping the container in an upright position
- 3 or 4 applications per day used to be anough, but it can be used as often as necessary
Propyleneglycol, water, Polysorbate 80, Glycyrrhizinic Acid, Sodium Benzoate, Potassium Sorbate, TEA

