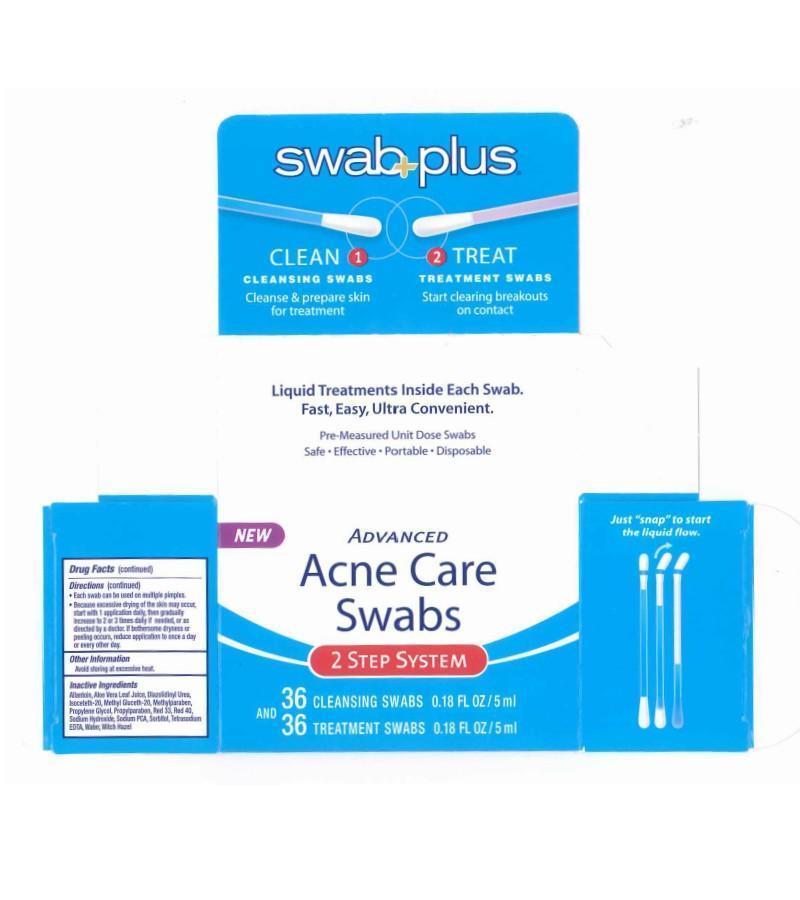
Drug Facts
Active Ingredients
Advance Acne Care Swabs step 1
Silver 0.001%
Advance Acne Care Swabs Step 2
Salicylic Acid 2.0%
Uses
When using this product. Avoid contact with eyes. If contact occures. flush throughly with water. Using other topical acne medications at teh same time or immediately following use this product may increase dryness or irrtation of the skin. If this occures, only one medication shoukld be used unless directed by a doctor. Do not insert in teh canal.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Do not use if label seal is broken prior to purchase.Keep swabs in original container when not in use. Treatment Swabs have a red color band.
- Use Cleansinh swab to cleanse the skin throughly before applying medication.
- Hold the swab vertically, with the color ring band tip upwards.
- Bend the tip at the color band to one side until it snaps. Medicine will flow to theother end.
- Cover the entire affected area with a thin layer 1 to 3 times daily. Discard swab after use.
- Each swab can be used on multipe pimples.
- Because excesssive drying of the skin may occur start with1 application daiky then gradually increase to 2 or 3 times daily if needed, or as direcvted by a doctor. If bothersome dryness or peeling occurs, reduce application to once a day or every othr day.