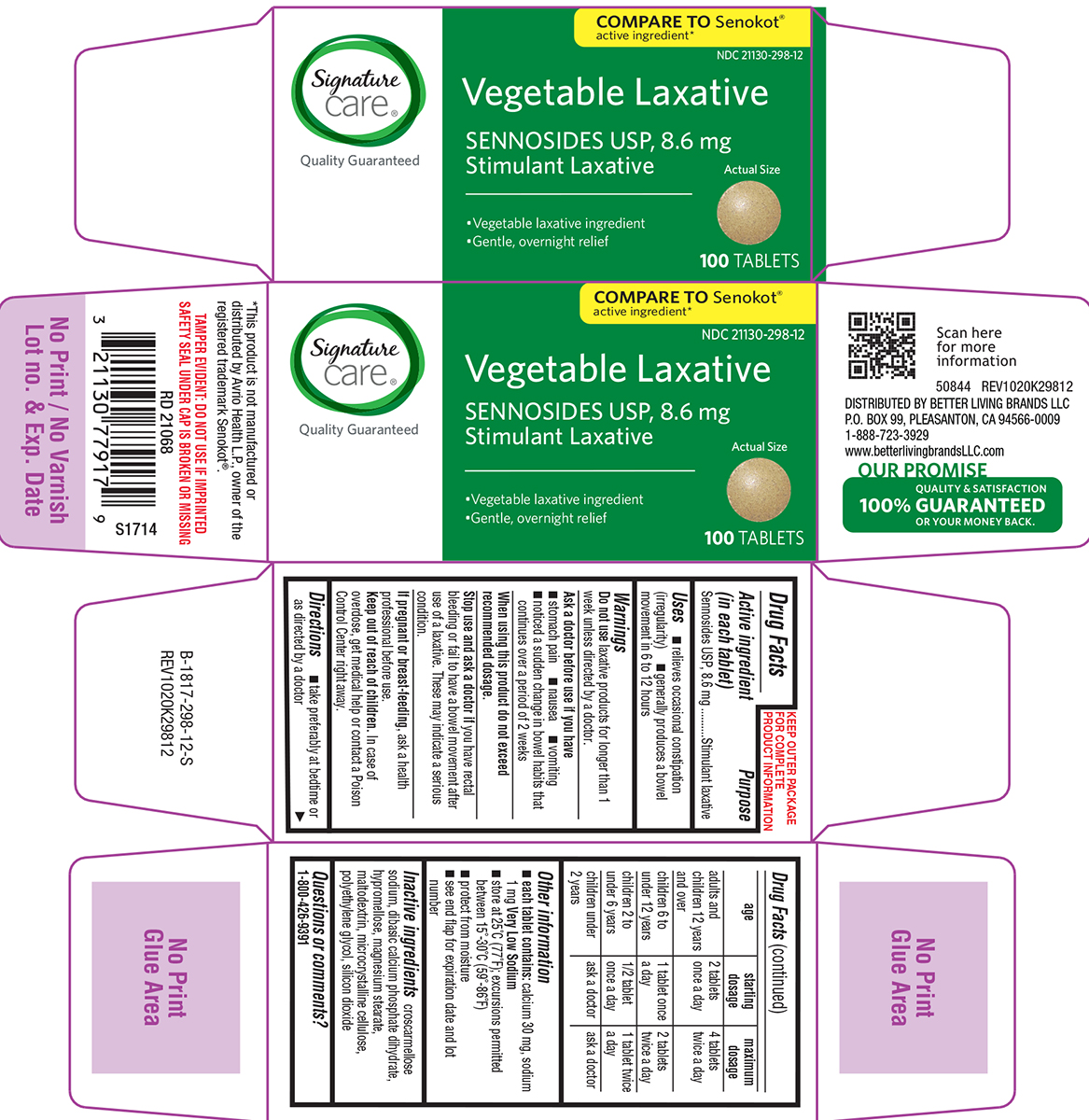
Uses
- relieves occasional constipation (irregularity)
- generally produces a bowel movement in 6 to 12 hours
Warnings
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that continues over a period of 2 weeks
Directions
- take preferably at bedtime or as directed by a doctor
| age | starting dosage | maximum dosage |
| adults and children 12 years and over | 2 tablets once a day | 4 tablets twice a day |
| children 6 to under 12 years | 1 tablet once a day | 2 tablets twice a day |
| children 2 to under 6 years | 1/2 tablet once a day | 1 tablet twice a day |
| children under 2 2 years | ask a doctor | ask a doctor |
Other Information
- each tablet contains: calcium 30 mg, sodium 1 mg Very Low Sodium
- store at 25ºC (77ºF); excursions permitted between 15°-30°C (59°-86°F)
- protect from moisture
- see end flap for expiration date and lot number
Inactive ingredients
croscarmellose sodium, dibasic calcium phosphate dihydrate, hypromellose, magnesium stearate, maltodextrin, microcrystalline cellulose, polyethylene glycol, silicon dioxide
Principal Display Panel
Signature
care®
Quality Guaranteed
COMPARE TO Senokot®
active ingredient*
NDC 21130-298-12
Vegetable Laxative
SENNOSIDES USP, 8.6 mg
Stimulant Laxative
• Vegetable laxative ingredient
• Gentle, overnight relief
Actual Size
100 TABLETS
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
*This product is not manufactured or
distributed by Avrio Health L.P., owner of the
registered trademark Senokot®.
50844 REV1020K29812
DISTRIBUTED BY BETTER LIVING BRANDS LLC
P.O. BOX 99, PLEASANTON, CA 94566-0009
1-888-723-3929
www.betterlivingbrandsLLC.com
OUR PROMISE
QUALITY & SATISFACTION
100% GUARANTEED
OR YOUR MONEY BACK.
44-298