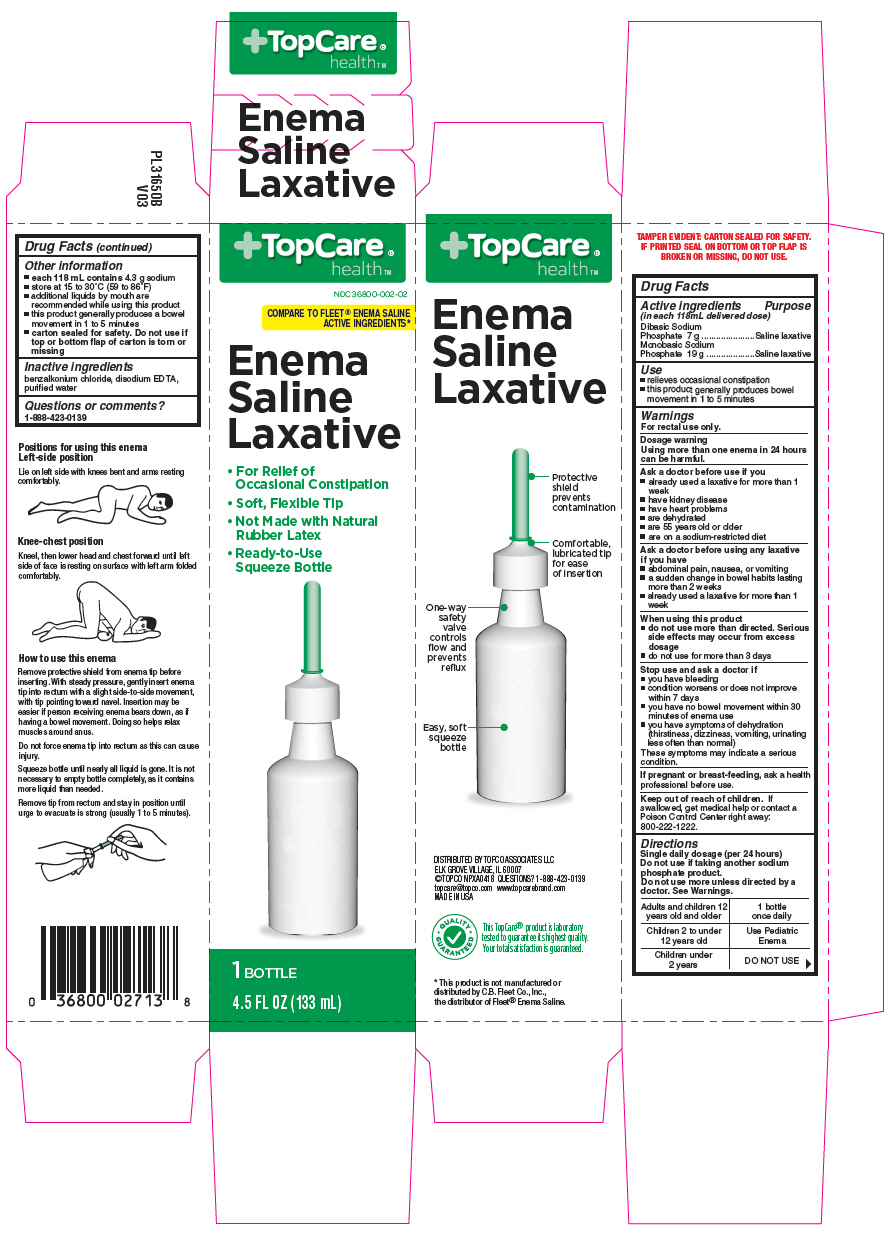
Use
- relieves occasional constipation
- this product generally produces bowel movement in 1 to 5 minutes
Warnings
For rectal use only.
Ask a doctor before use if you
- already used a laxative for more than 1 week
- have kidney disease
- have heart problems
- are dehydrated
- are 55 years old or older
- are on a sodium-restricted diet
Ask a doctor before using any laxative if you have
- abdominal pain, nausea, or vomiting
- a sudden change in bowel habits lasting more than 2 weeks
- already used a laxative for more than 1 week
When using this product
- do not use more than directed. Serious side effects may occur from excess dosage
- do not use for more than 3 days
Stop use and ask a doctor if
- you have bleeding
- condition worsens or does not improve within 7 days
- you have no bowel movement within 30 minutes of enema use
- you have symptoms of dehydration (thirstiness, dizziness, vomiting, urinating less often than normal)
These symptoms may indicate a serious condition.
Directions
Single daily dosage (per 24 hours)
Do not use if taking another sodium phosphate product.
Do not use more unless directed by a doctor. See Warnings.
| Adults and children 12 years old and older | 1 bottle once daily |
| Children 2 to under 12 years old | Use Pediatric Enema |
| Children under 2 years | DO NOT USE |
Other information
- each 118 mL contains 4.3 g sodium
- store at 15 to 30°C (59 to 86°F)
- additional liquids by mouth are recommended while using this product
- this product generally produces a bowel movement in 1 to 5 minutes
- carton sealed for safety. Do not use if top or bottom flap of carton is torn or missing