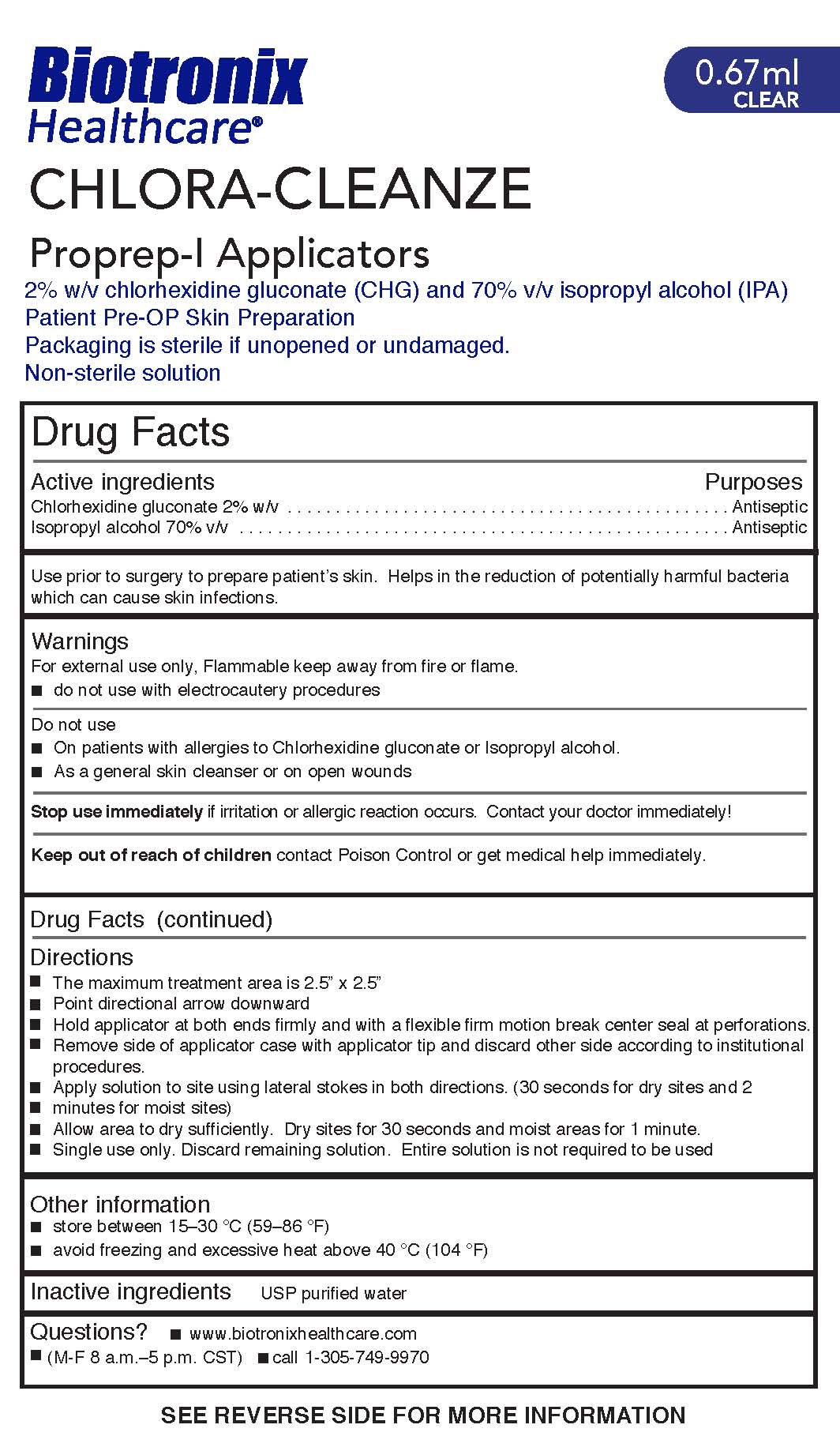
Warnings
For external use only. Flammable keep away from fire or flame.
- do not use with electrocautery procedures
Do not use
- On patients with allergies to Chlorhexidine gluconate or Isopropyl alcohol
- As a general skin cleanser or on open wounds
Directions
- The maximum treatment area is 2.5” x 2.5”
- Point directional arrow downward
- Hold applicator at both ends firmly and with a flexible firm motion break center seal at perforations.
- Remove side of applicator case with applicator tip and discard other side according to institutional
procedures.
- Apply solution to site using lateral stokes in both directions. (30 seconds for dry sites and 2
minutes for moist sites)
- Allow area to dry sufficiently. Dry sites for 30 seconds and moist areas for 1 minute.
- Single use only. Discard remaining solution. Entire solution is not required to be used
Use prior to surgery to prepare patient’s skin. Helps in the reduction of potentially harmful bacteria which can cause skin infections.