Uses
for the temporary relief of redness and itching of the eye(s) due to:
- ragweed
- pollen
- grass
- animal dander and hair
Do not use
- if you are sensitive to any ingredient in this product
- if this solution changes color or becomes cloudy
Ask a doctor before use if you have
- narrow angle glaucoma
- heart disease
- high blood pressure
- trouble urinating
When using this product
- pupils may become enlarged temporarily causing light sensitivity
- overuse may cause more eye redness
- remove contact lenses before using
- to avoid contamination, do not touch tip of container to any surface
- replace cap after each use
Stop use and ask a doctor if
- you feel eye pain
- changes in vision occur
- redness or irritation of the eye(s) gets worse or lasts more than 72 hours
Keep out of the reach of children.
If swallowed, get medical help or contact a Poison Control Center right away. Accidental swallowing by infants and children may lead to coma and marked reduction in body temperature.
Directions
- adults and children 6 years and over: put 1 or 2 drops in the affected eye(s) up to 4 times daily
- children under 6 years: ask a doctor
Inactive ingredients
benzalkonium chloride, boric acid, edetate disodium, purified water, sodium borate, sodium chloride, sodium hydroxide and/or hydrochloric acid
PRINCIPAL DISPLAY PANEL
$3 Coupon Inside
NAPHCON A®
Naphazoline Hydrochloride 0.025% and Pheniramine Maleate 0.3%
Ophthalmic Solution
Redness Reliever and Antihistamine
RELIEVES ITCHING & REDNESS
EYE DROPS
STERILE 15 mL (0.5 FL OZ)
TAMPER EVIDENT: For your protection, this bottle has a seal imprinted with the word “Alcon” around the neck. Do not use if seal is damaged or missing at time of purchase.
Actual Size
Combines a redness reliever and an antihistamine for the temporary relief of itchy, red eyes.
SEE COUPON INSIDE
ALCON LABORATORIES, INC.
6201 South Freeway
Fort Worth, TX 76134 USA
Alcon
LOT:
EXP.:
300055595-0122

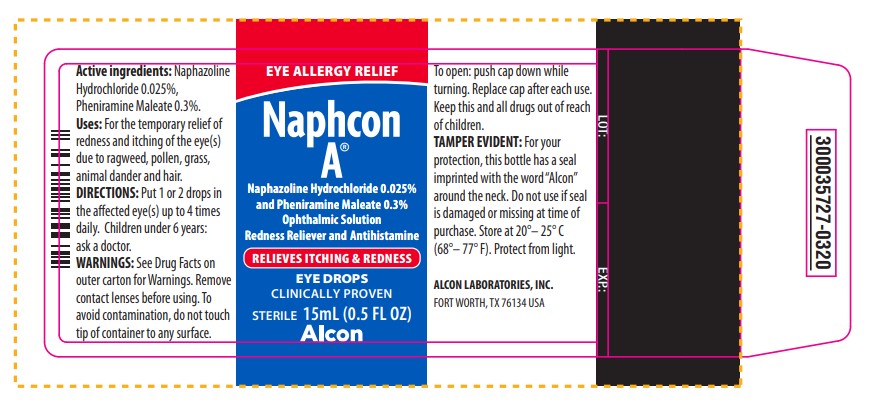
EYE ALLERGY RELIEF
NAPHCON A®
Naphazoline Hydrochloride 0.025% and Pheniramine Maleate 0.3%
Ophthalmic Solution
Redness Reliever and Antihistamine
RELIEVES ITCHING & REDNESS
EYE DROPS
CLINICALLY PROVEN
STERILE 15 mL (0.5 FL OZ)
Alcon
Active ingredients: Naphazoline
Active ingredients: Naphazoline Hydrochloride 0.025%, Pheniramine Maleate 0.3%.
Uses: For the temporary relief of redness and itching of the eye(s) due to ragweed, pollen, grass, animal dander and hair.
DIRECTIONS: Put 1 or 2 drops in the affected eye(s) up to 4 times daily. Children under 6 years: ask a doctor.
WARNINGS: See Drug Facts on outer carton for Warnings. Remove contact lenses before using. To avoid contamination, do not touch tip of container to any surface.
To open: push cap down while turning. Replace cap after each use.
Keep this and all drugs out of reach of children.
TAMPER EVIDENT: For your protection, this bottle has a seal imprinted with the word “Alcon” around the neck. Do not use if seal is damaged or missing at time of purchase. Store at 20° - 25°C (68° - 77°F). Protect from light.
ALCON LABORATORIES, INC.
FORT WORTH, TX 76134 USA
LOT: EXP:
300035727-0320
PRINCIPAL DISPLAY PANEL
Convenient • Take Anywhere
EYE ALLERGY RELIEF
POCKET SIZE
NAPHCON A®
Naphazoline Hydrochloride 0.025% and Pheniramine Maleate 0.3%
Ophthalmic Solution
Redness Reliever and Antihistamine
Relieves Itching & Redness
EYE DROPS
CLINICALLY PROVEN
STERILE Two 5 mL (0.17 FL OZ) Bottles
TAMPER EVIDENT: For your protection, this bottle has a seal imprinted with the word “Alcon” around the neck. Do not use if seal is damaged or missing at time of purchase.
ACTUAL SIZE
Combines a redness reliever and an antihistamine for the temporary relief of itchy, red eyes.
ACTUAL FILL
ALCON LABORATORIES, INC.
6201 South Freeway
Fort Worth, TX 76134
ALCON
LOT:
EXP.:
9017143-0419

PRINCIPAL DISPLAY PANEL
EYE ALLERGY RELIEF
NAPHCON A®
Naphazoline Hydrochloride 0.025% and Pheniramine Maleate 0.3% Ophthalmic Solution
Redness Reliever and Antihistamine
RELIEVES ITCHING & REDNESS
EYE DROPS
CLINICALLY PROVEN
STERILE 5mL (0.17 FL OZ )
Active ingredients: Naphazoline Hydrochloride 0.025%, Pheniramine Maleate 0.3%.
Uses: For the temporary relief of redness and itching of the eye(s) due to ragweed, pollen, grass, animal dander and hair.
DIRECTIONS: Put 1 or 2 drops in the affected eye(s) up to 4 times daily. Children under 6 years: ask a doctor.
WARNINGS: See Drug Facts on outer carton for Warnings. Remove contact lenses before using. To avoid contamination, do not touch tip of container to any surface. To open: push cap down while turning. Replace cap after each use. Keep this and all drugs out of reach of children.
TAMPER EVIDENT: For your protection, this bottle has a seal imprinted with the word “Alcon” around the neck. Do not use if seal is damaged or missing at time of purchase.
Store at 20° - 25°C (68° - 77°F). Protect from light.
ALCON LABORATORIES, INC.
FORT WORTH, TX 76134 USA
LOT
EXP:
300035726-0320
