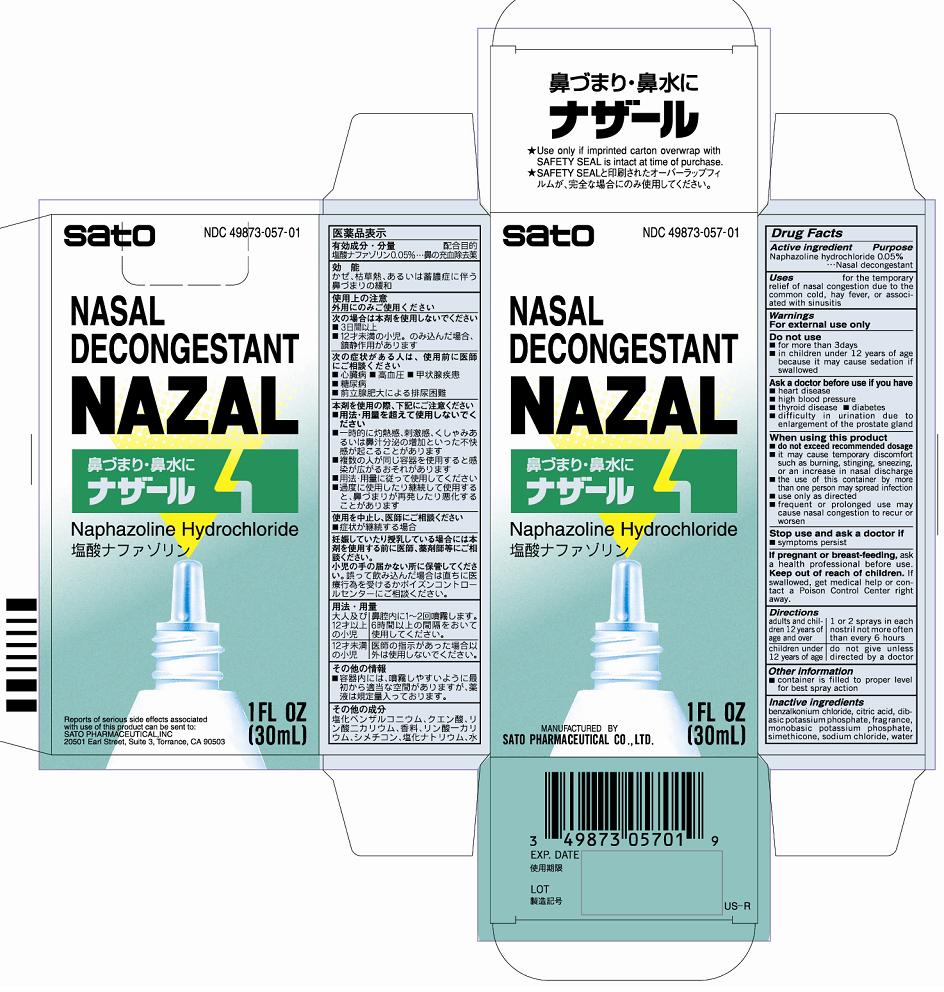
Uses for the temporary relief of nasal congestion due to the common cold, hay fever, or associated with sinusitis
Warnings
For external use only
Do not use
■for more than 3 days
■in children under 12 years of age because it may cause sedation if swallowed
Ask a doctor before use if you have
■heart disease
■high blood pressure
■thyroid disease ■diabetes
■difficulty in urination due to enlargement of the prostate gland
When using this product
■do not exceed recommended dosage
■if may cause temporary discomfort such as burning, stinging, sneezing, or an increase in nasal discharge
■the use of this container by more than one person may spread infection
■use only as directed
■frequent or prolonged use may cause nasal congestion to recur or worsen
Directions
Adults and children 12 years of age and older - 1 or 2 sprays in each nostril not more often than every 6 hours
Children under 12 years of age - do not give unless directed by a doctor