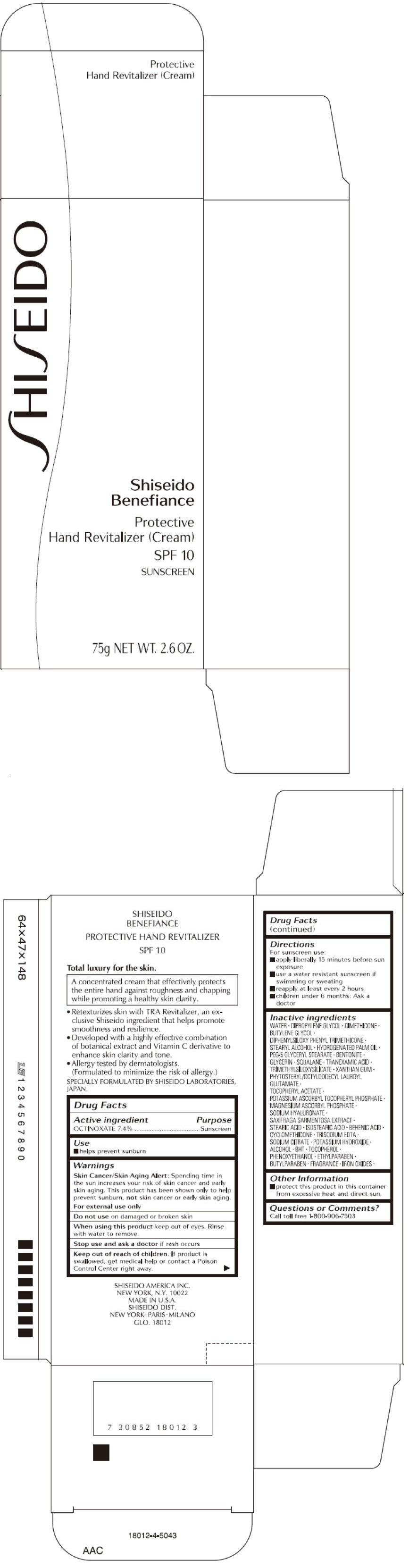
BENEFIANCE PROTECTIVE HAND REVITALIZER- octinoxate cream
SHISEIDO AMERICA INC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
OCTINOXATE 7.4%
Warnings
Skin Cancer/Skin Aging Alert
Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to prevent sunburn, not skin cancer or early skin aging.
For external use only
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs
Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- children under 6 months: Ask a doctor
Inactive Ingredients
WATER, DIPROPYLENE GLYCOL, DIMETHICONE, BUTYLENE GLYCOL, DIPHENYLSILOXY PHENYL TRIMETHICONE, STEARYL ALCOHOL, HYDROGENATED PALM OIL, PEG-5 GLYCERYL STEARATE, BENTONITE, GLYCERIN, SQUALANE, TRANEXAMIC ACID, TRIMETHYLSILOXYSILICATE, XANTHAN GUM, PHYTOSTERYL/OCTYLDODECYL LAUROYL GLUTAMATE, TOCOPHERYL ACETATE, POTASSIUM ASCORBYL TOCOPHERYL PHOSPHATE, MAGNESIUM ASCORBYL PHOSPHATE, SODIUM HYALURONATE, SAXIFRAGA SARMENTOSA EXTRACT, STEARIC ACID, ISOSTEARIC ACID, BEHENIC ACID, CYCLOMETHICONE, TRISODIUM EDTA, SODIUM CITRATE, POTASSIUM HYDROXIDE, ALCOHOL, BHT, TOCOPHEROL, PHENOXYETHANOL, ETHYLPARABEN, BUTYLPARABEN, FRAGRANCE, IRON OXIDES,
Other information
- protect this product in this container from excessive heat and direct sun.
Questions or comments?
Call toll free 1-800-906-7503
PRINCIPAL DISPLAY PANEL - 75g Tube Carton
SHISEIDO
Shiseido
Benefiance
Protective
Hand Revitalizer (Cream)
SPF 10
SUNSCREEN
75g NET WT. 2.6 OZ.
SHISEIDO AMERICA INC.