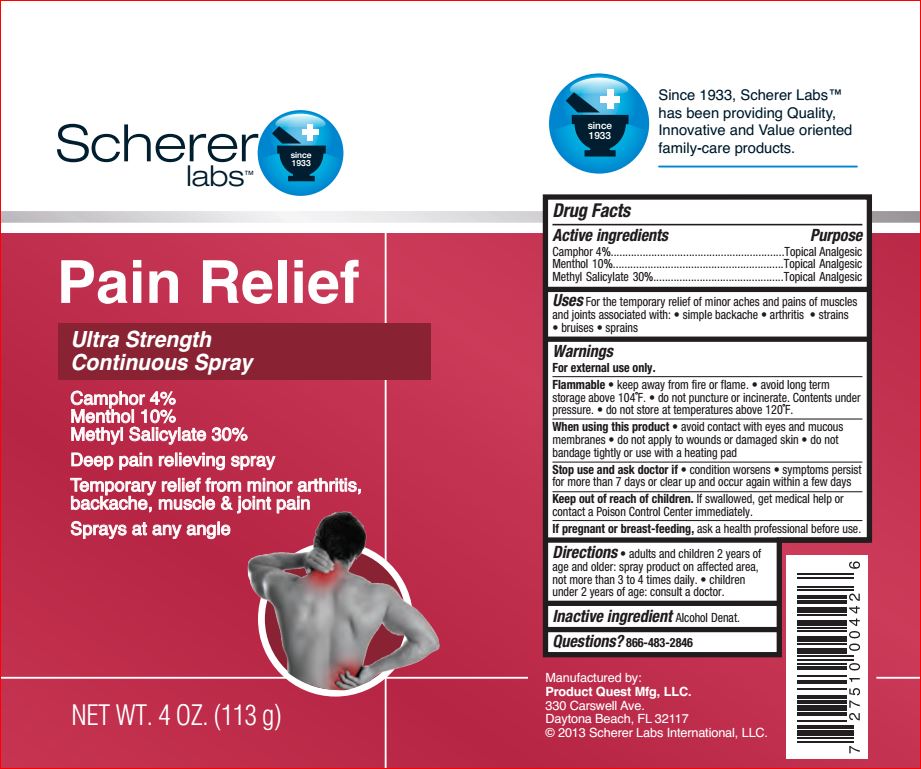
Active ingredients Purpose
Camphor – 4.00% Topical Analgesic
Menthol – 10.00% Topical Analgesic
Methyl Salicylate – 30.00% Topical Analgesic
Uses
- temporarily relieves minor pain associated with:
- arthritis
- simple backache
- muscle strains
- bruises
- muscle sprains
Warnings
For external use only
Flammable
• keep away from fire or flame. • avoid long term
storage above 104˚F. • do not puncture or incinerate. Contents under
pressure. • do not store at temperatures above 120˚F
• do not use while smoking or near heat or flame
• avoid long term storage above 104F
• do not puncture or incinerate. Contents under pressure
• do not store at temperature above 120F
Stop use and ask a doctor if
• condition worsens
• symptoms last more than 7 days or clear up and occur again within a few days
Keep out of reach of the children
If swallowed, get medical help or contact a Poison Control Center right away