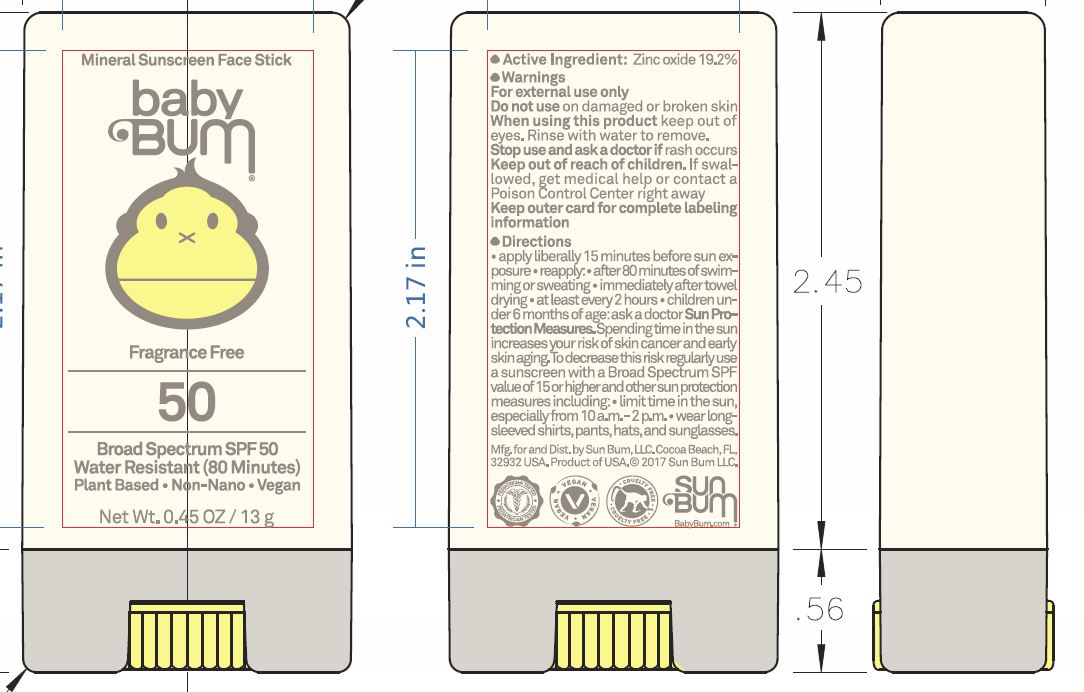
Uses
• helps prevent sunburn • if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
• apply liberally 15 minutes before sun exposure reapply: • after 80 minutes of swimming or sweating • immediately after towel drying • at least every 2 hours • children under 6 months of age: ask a doctor Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: • limit time in the sun, especially from 10 a.m.-2 p.m. • wear long-sleeved shirts, pants, hats, and sunglasses
Other information
• protect this product from excessive heat and direct sun • keep this outer card for complete labeling information
Inactive ingredients
cocos nucifera (coconut) oil, persea gratissima (avocado) oil, limnanthes alba (meadowfoam) seed oil, linum usitatissimum (linseed) seed oil, oryza sativa (rice bran) wax, euphorbia cerifera (candelilla) wax, butyloctyl salicylate, copernicia cerifera (carnauba) wax, ozokerite, butyrospermum parkii (shea butter), oryzanol, fragrance, theobroma cacao (cocoa) seed butter, carthamus tinctorius (safflower) seed oil, jojoba esters, tocopherol, bisabolol