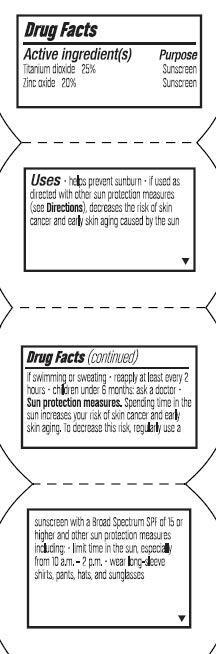
Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
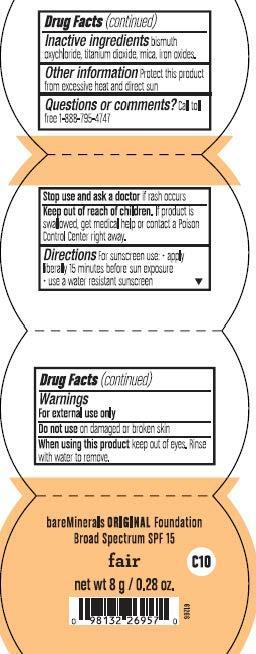
Warnings
- Skin cancer/skin aging alert: spending time in the sun increases your risk of cancer and early aging
- This product has been shown only to help prevent sunburn, not skin cancer or early aging
- For External use only
- Do not use on damage or broken skin
- When using this product keep out of eyes. Rinse with water to remove
- Stop use and ask doctor if rash occurs
Directions
Apply liberally 15 minutes before sun exposure
Use a water-resistant sunscreen if swimming or sweating
Reapply at least every 2 hours
Sun protection measures. Spending time in the sun increases your risk of skin cancer or early skin aging. To decrease this risk, regularly use a a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
Limit time in the sun, especially from 10 a.m. - 2 p.m.
Wear long sleeve shirts, pants, hats, and sunglasses
Children under 6 month: ask a doctor