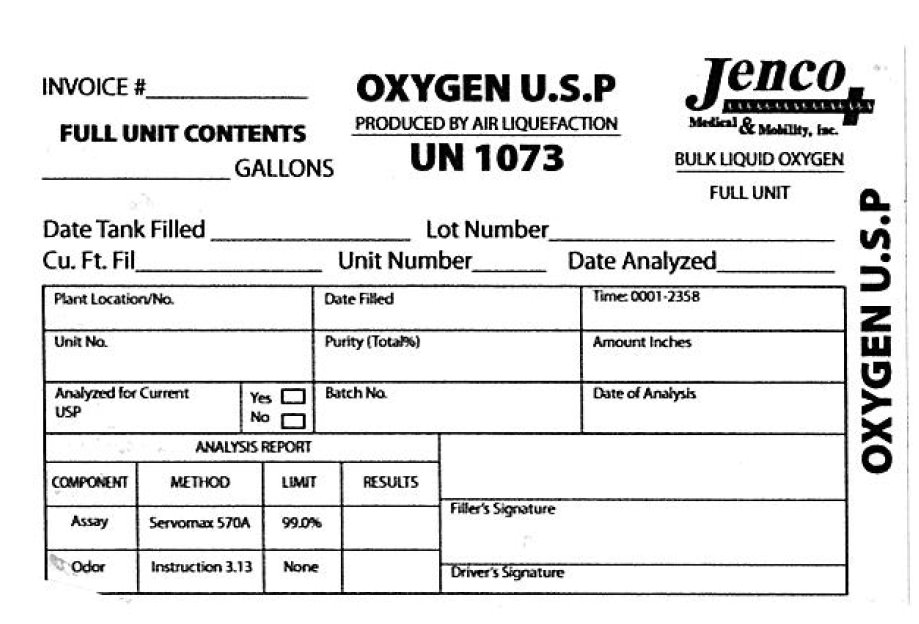
Invoice OXYGEN U.S.P Jenco Medical and Mobility
Full Unit Contents Produced By Air Liquefaction Bulk Liquid Oxygen
Gallons UN 1073 Full Unit
Date Tank Filled Lot Number
Cu. Ft. Fil Unit Number Date Analyzed
Plant Location /No. Date Filled Time:0001-2358
Unit No. Purity Amount Inches
Analyzed for Current USP Batch No Date of Analysis
Analysis Report
Component Method Limit Results
Assay Servomax 570A 99.0 Percent
Odor Instruction 3.13 none
Filler's Signature
Driver's Signature