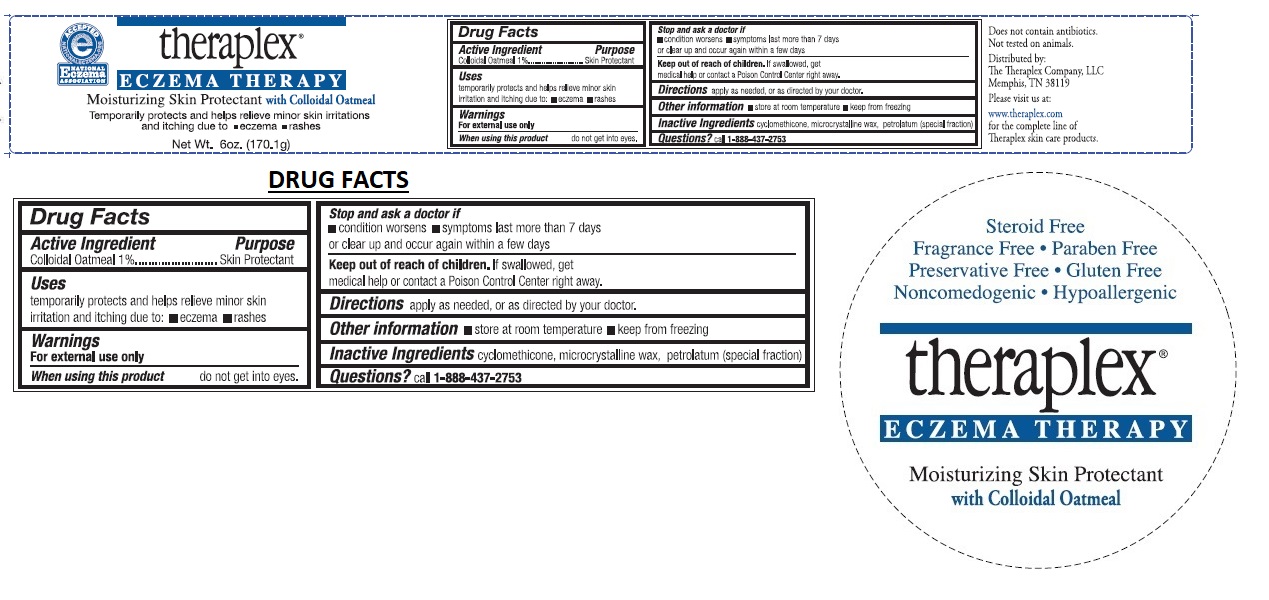
THERAPLEX ECZEMA THERAPY MOISTURIZING SKIN PROTECTANT- colloidal oatmeal lotion
The Theraplex Company, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredients
Colloidal Oatmeal 1%
Uses
temporarily protects and helps relieve minor skin irritation and itching due to: • eczema • rashes
Warnings
For external use only.
When using this product do not get into eyes.
Stop use and ask a doctor if
• condition worsens • symptoms last more than 7 days or clear up and occur again within a few days.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
apply as needed, or as directed by your doctor.
Other information
• store at room temperature • keep from freezing
Inactive Ingredients
cyclomethicone,microcrystalline wax, petrolatum(special fraction)
Questions?
call 1-888-437-2753
Does not contain antibiotics.
Not tested on animals.
Distributed by:
The Theraplex Company, LLC
Memphis,TN 38119
Steroid Free
Fragrance Free
Paraben Free
Preservative Free
Gluten Free
Noncomedogenic
Hypoallergenic
Packaging