Drug Facts Active Ingredients
All active ingredients are HPUS*
Arncia 4X, Causticum 4X, Rhamnus Calif. 4X, Ruta 4X, Taraxacum 4X,
Belladonna 4X, Cimicifuga 4X, Gnaphalium 4X, Hypericum Perf. 4X, Jatropha 8X, Veratrum 8X
Cina 8X, Ignatia 4X, Kali Brom. 4X, Kali Carb. 4X, Lycopodium 4X, Scutellaria 4X, Valeriana Off. 4X
*HPUS indicates the active ingredients are in the official Homeopathic Pharmacopeia of the United States.
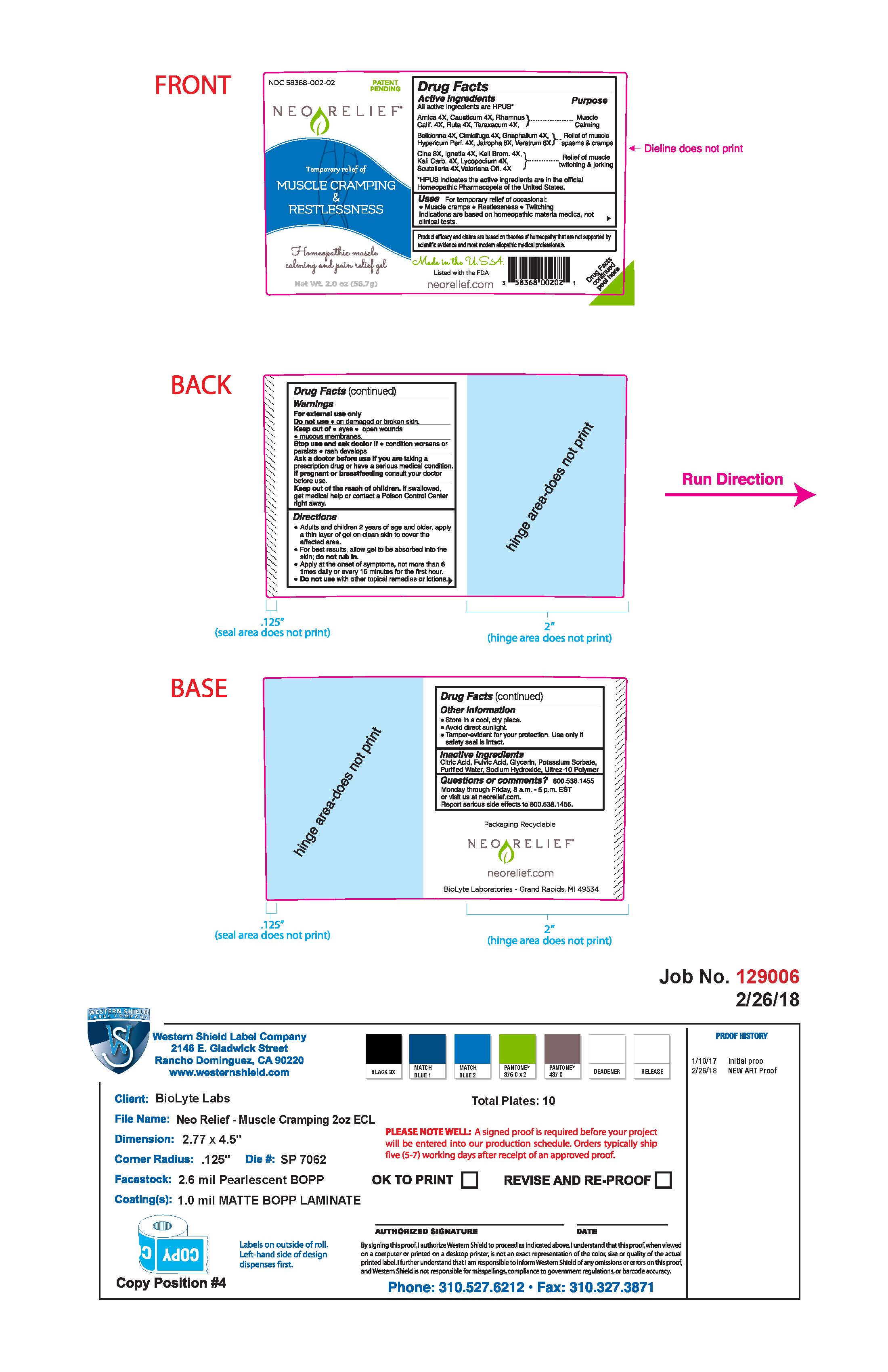
Purpose
For the temporary relief of occasional:
Muscle cramps, restlessness, twiching
Indications are based on homeopathic materia medica, not clinical tests.
Warnings
For external use only
Do not use on damaged or broken skin.
Keep out of eyes, open wounds, mucous membranes
Warnings
Ask a doctor before use if you are taking a prescription drug or have a serious medical condition.
Warnings
Keep out of the reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Adults and children 2 years of age and older, apply a thin layer of gel on clean skin to cover the affected area.
For best results, allow gel to be absorbed into the skin; do not rub in.
Apply at the onset of symptoms, not more than 6 times daily or every 15 minutes for the first hour.
Do not use with other topical remedies or lotions.
Other Information
Store in a cool, dry place
Avoid direct sunlight
Tamper-evident for your protection. Use only if safety seal is intact.
Inactive Ingredients
Citric Acid, Fulvic acid, Glycerin, Potassium Sorbate, Purified Water, Sodium Hydroxide, Ultrez-10 Polymer
Questions or comments?
800.538.1455
Monday through Friday 8 a.m. - 5 p.m. EST or visit us at neorelief.com
Report serious side effects to 800.538.1455
Additional Label Content
Required FTC Disclosure: Product efficacy and claims are based on theories of homeopathy that are not supported by scientific evidence and most modern allopathic medical professionals.
Made in the USA
Listed with the FDA
(UPC code: 3 58368 00202 1)
Drug Facts continued peel here
Packaging Recyclable
neorelief.com
BioLyte Laboratories- Grand Rapids, MI 49534