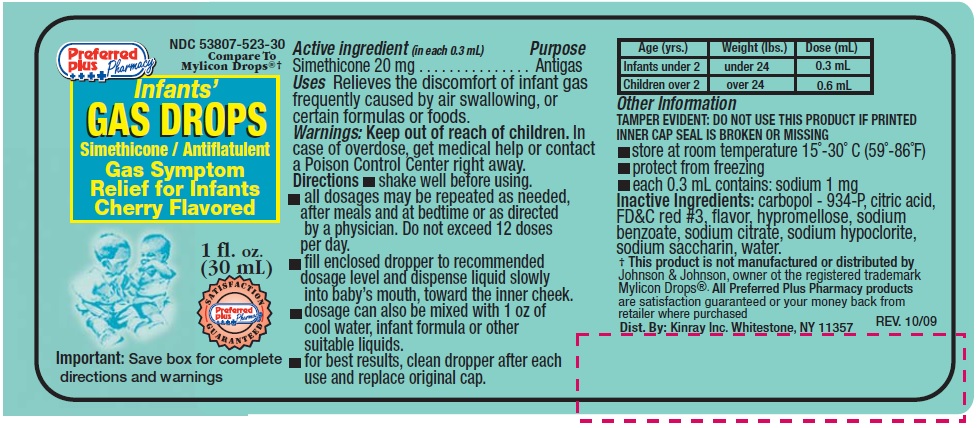
Uses
Relieves the discomfort of gas frequently caused by air swallowing, or certain formulas or foods.
Directions
- •
- shake well before using
- •
- all dosages may be repeated as needed, after meals and at bedtime or as directed by a physician. Do not exceed 12 doses per day.
- •
- fill enclosed dropper to recommended dosage level and dispense liquid slowly into baby's mouth, toward the inner cheek
- •
- dosage can also be mixed with 1 oz.of cool water, infant formula or other suitable liquids
- •
- for best results, clean dropper after each use and replace original cap.
| Age (yrs) | Weight (lbs) | Dose (ml) |
|---|---|---|
| Infants under 2 | Under 24 | 0.3 mL |
| Children over 2 | Over 24 | 0.6 mL |
Other Information
- TAMPER EVIDENT: DO NOT USE THIS PRODUCT IF PRINTED INNER CAP SEAL IS BROKEN OR MISSING
- •
- Store at room temperature 15° - 30°C (59 - 86°F)
- •
- protect from freezing
- •
- each 0.3 mL contains: sodium 1 mg