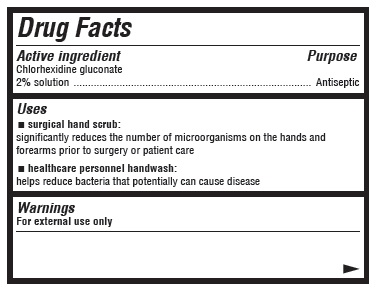
Uses
- surgical hand scrub: significantly reduces the number of microorganisms on the hands and forearms prior to surgery or patient care
- healthcare personnel handwash: helps reduce bacteria that potentially can cause disease
Warnings
For external use only
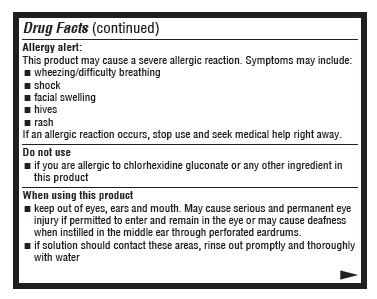
Allergy alert:
This product may cause a severe allergic reaction. Symptoms may include:
- wheezing/difficulty breathing
- shock
- facial swelling
- hives
- rash
If an allergic reaction occurs, stop use and seek medical help right away.
When using this product
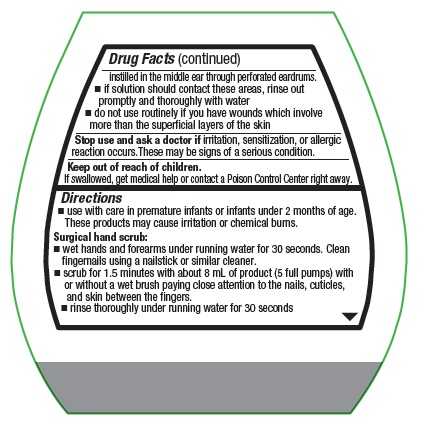
- keep out of eyes, ears, and mouth. May cause serious and permanent eye injury if placed or kept in the eye during surgical procedures or may cause deafness when instilled in the middle ear through perforated eardrums.
- if solution should contact these areas, rinse out promptly and thoroughly with water
- do not use routinely if you have wounds which involve more than the superficial layers of the skin
Directions
- use with care in premature infants or infants under 2 months of age. These products may cause irritation or chemical burns.
Surgical hand scrub:
- wet hands and forearms under running water for 30 seconds. Clean fingernails using a nailstick or similar cleaner.
- scrub for 1.5 minutes with about 8 mL of product with or without a wet brush paying close attention to the nails, cuticles, and skin between the fingers
- rinse thoroughly under running water for 30 seconds
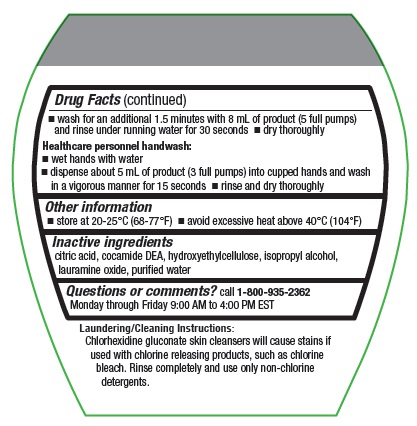
- wash for an additional 1.5 minutes with 8 mL of product and rinse under running water for 30 seconds
- dry thoroughly
Healthcare personnel handwash:
- wet hands with water
- dispense about 5 mL of product into cupped hands and wash in a vigorous manner for about 15 seconds
- rinse and dry thoroughly
Inactive ingredients
citric acid, cocamide DEA, hydroxyethylcellulose, isopropyl alcohol, lauramine oxide, purified water
Laundering/Cleaning Instructions: Chlorhexidine gluconate skin cleansers will cause stains if used with chlorine releasing products. Rinse completely and use only non-chlorine detergents.
Warning: This product can expose you too coconut oil diethanolamine condensate (cocamide diethanolamine), whichs is known to the State of California to cause cancer. For more information go to www.P65Warnings.ca.gov
Package Label
NDC 63868-280-02
Chlorhexidine Gluconate 2% Solution
Antiseptic
Distributed by:
C,D,M,A,Inc.
Novi, MI 48376 USA
qualitychoice.com
2QC02BTLLBLB
Quality Choice ®
Net Wt 2 fl oz (60 mL)




 .
.
NDC 63868-280-08
QUALITY CHOICE
Antiseptic Skin Cleanser
Antiseptic/Antimicrobial Skin Cleaner
Chlorhexidine Gluconate 2% Solution
Reduces Bacteria on the Skin
FOR EXTERNAL USE ONLY
TRICLOSAN FREE
FDA APPROVED
8.5 fl oz (250 mL)
2QC85BTLLBLFB