Uses
- for the temporary relief of burning and irritation due to dryness of the eye
- for use as a lubricant to prevent further irritation or to relieve dryness of the eye
When using this product
- remove contact lenses before using
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
Stop use and ask a doctor if you are experiencing any of the following:
- eye pain
- changes in vision
- continued redness or irritation of the eye
- condition worsens or persist for more than 72 hours
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Pull down the lower lid of the affected eye and apply a small amount (one-fourth inch) of ointment to the inside of the eyelid.
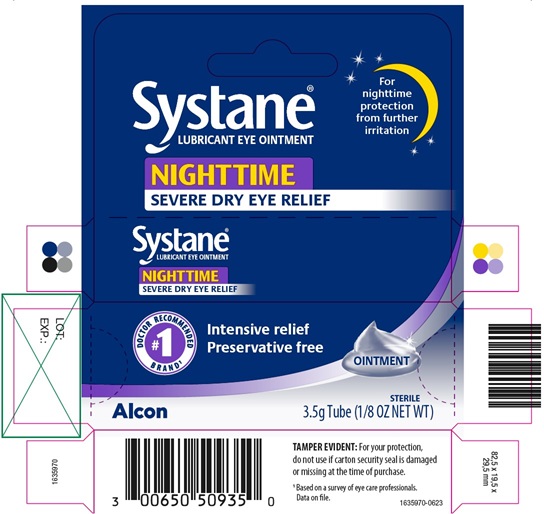
PRINCIPAL DISPLAY PANEL
Systane®
LUBRICANT EYE OINTMENT
NIGHTTIME
SEVERE DRY EYE RELIEF
For nighttime protection from further irritation
Systane®
LUBRICANT EYE OINTMENT
NIGHTTIME
SEVERE DRY EYE RELIEF
#1 DOCTOR RECOMMENDED BRAND1
Intensive relief
Preservative free
OINTMENT
Alcon
STERILE
3.5 g Tube (1/8 OZ NET WT)
TAMPER EVIDENT: For your protection,
do not use if carton security seal is damaged
or missing at the time of purchase.
1 Based on a survey of eye care professionals.
Data on file.
1635970-0623
LOT:
EXP.:
Systane®
LUBRICANT EYE OINTMENT
Alcon
Made in Belgium
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA