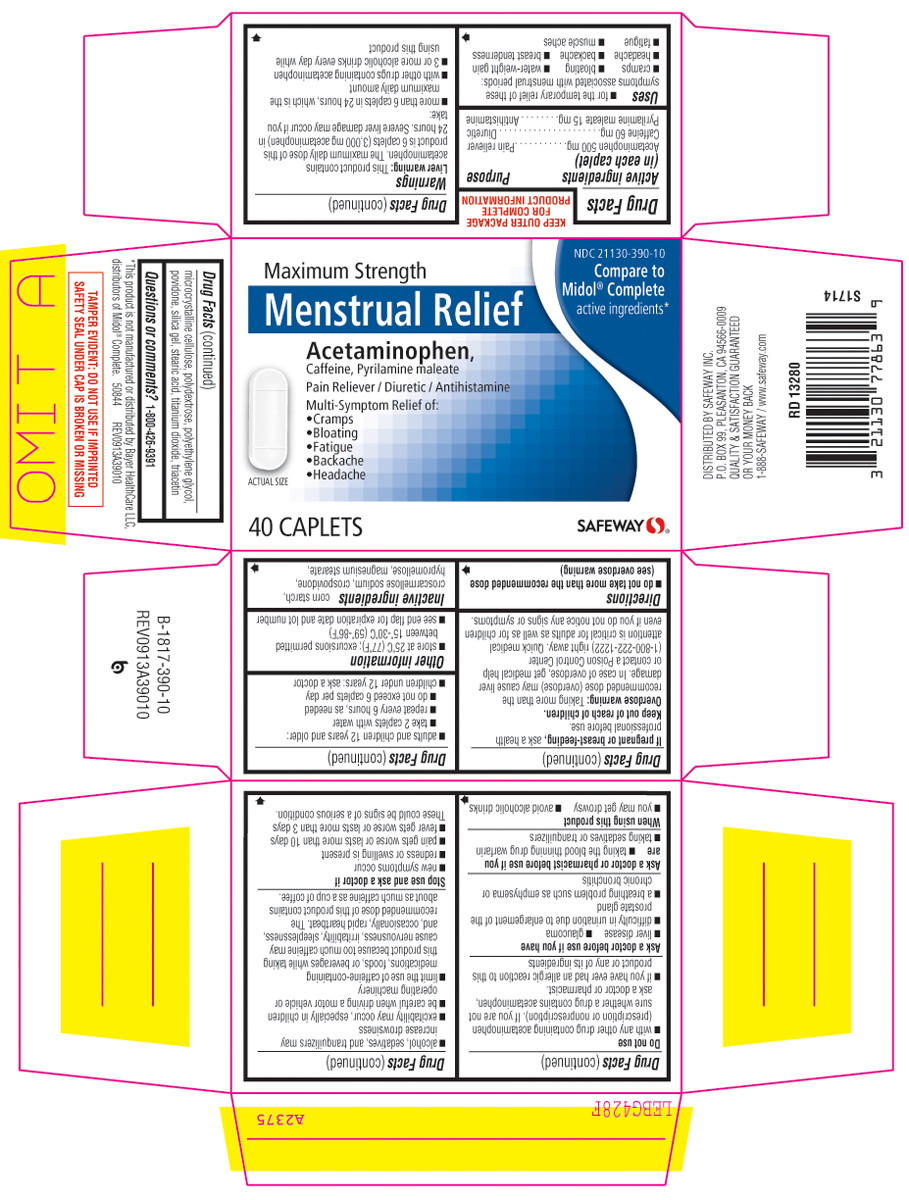
MENSTRUAL RELIEF MAXIMUM STRENGTH- acetaminophen, caffeine and pyrilamine maleate tablet, film coated
Better Living Brands, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Safeway 44-390-Delist
Uses
- for the temporary relief of these symptoms associated with menstrual periods:
- cramps
- bloating
- water-weight gain
- headache
- backache
- breast tenderness
- fatigue
- muscle aches
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 8 caplets in 24 hours, which is the maximum daily ammount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinnks every day while using this product
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Ask a doctor before use if you have
- difficulty in urination due to enlargement of the prostate gland
- liver disease
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
When using this product
- you may get drowsy
- be careful when driving a motor vehicle or operating machinery
- avoid alcoholic drinks
- excitability may occur, especially in children
- alcohol, sedatives and tranquilizers may increase drowsiness
- limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and, occasionally, rapid heartbeat. The recommended dose of this product contains about as much caffeine as a cup of coffee.
Directions
- do not take more than the recommended dose
(see Overdose warning) - adults and children 12 years and older: take 2 caplets with water. Repeat every 6 hours, as needed. Do not exceed 8 caplets in 24 hours.
- children under 12 years: do not use this adult product in children under 12 years of age; this will provide more than the recommended dose (overdose) and may cause liver damage
Other information
- store at controlled room temperature 15º-30ºC (59º-86ºF)
- see end flap for expiration date and lot number
Inactive ingredients
corn starch, croscarmellose sodium, crospovidone, hypromellose, magneium stearate, microcrystalline cellulose, polydextrose, polyethylene glycol, povidone, silica gel, stearic acid, titanium dioxide, triacetin
Principal Display Panel
NDC 21130-390-10
Compare to Model® Complete active ingredient†
Maximum Strength
Menstrual Relief
Acetaminophen,
Caffeine, Pyrilamine maleate
Pain Reliever / Stimulant / Diuretic
• Cramps
• Bloating
• Fatigue
• Backache
• Headache
40 TABLETS
SAFEWAY™
†This product is not manufactured or distributed by Pfizer Consumer Healthcare, distributors of Midol® Complete.
50844 REV0913A39010
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Safeway 44-390
| MENSTRUAL RELIEF
MAXIMUM STRENGTH
acetaminophen, caffeine and pyrilamine maleate tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Better Living Brands, LLC (009137209) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | PACK(21130-390) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | MANUFACTURE(21130-390) | |