REFILL 2- benzalkonium chloride, bacitracin zinc, neomycin sulfate, polymyxin b sulfate
CMC Group, Inc.
----------
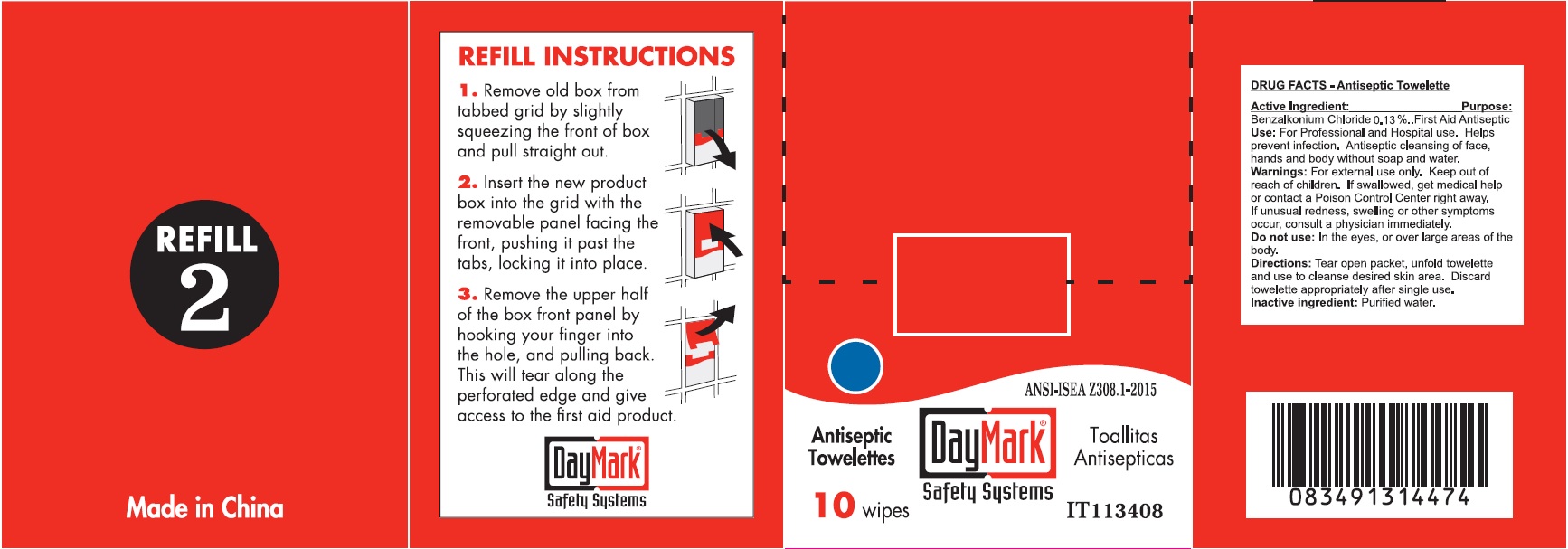
Drug Facts - Antiseptic Towelettes
Active Ingredient:
Benzalkonium Chloride 0.13%
Purpose:
First Aid Antiseptic
Use:
For Professional and Hospital use. Helps prevent infection. Antiseptic cleansing of face, hands and body without soap and water.
Warnings:
For external use only.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away. If unusual redness, swelling or other symptoms occur, consult a physician immediately.
Do not use:
In the eyes, or over large areas of the body.
Directions:
Tear open packet, unfold towelette and use to cleanse desired skin area. Discard towelette appropriately after single use.
Inactive ingredient:
Purified water.
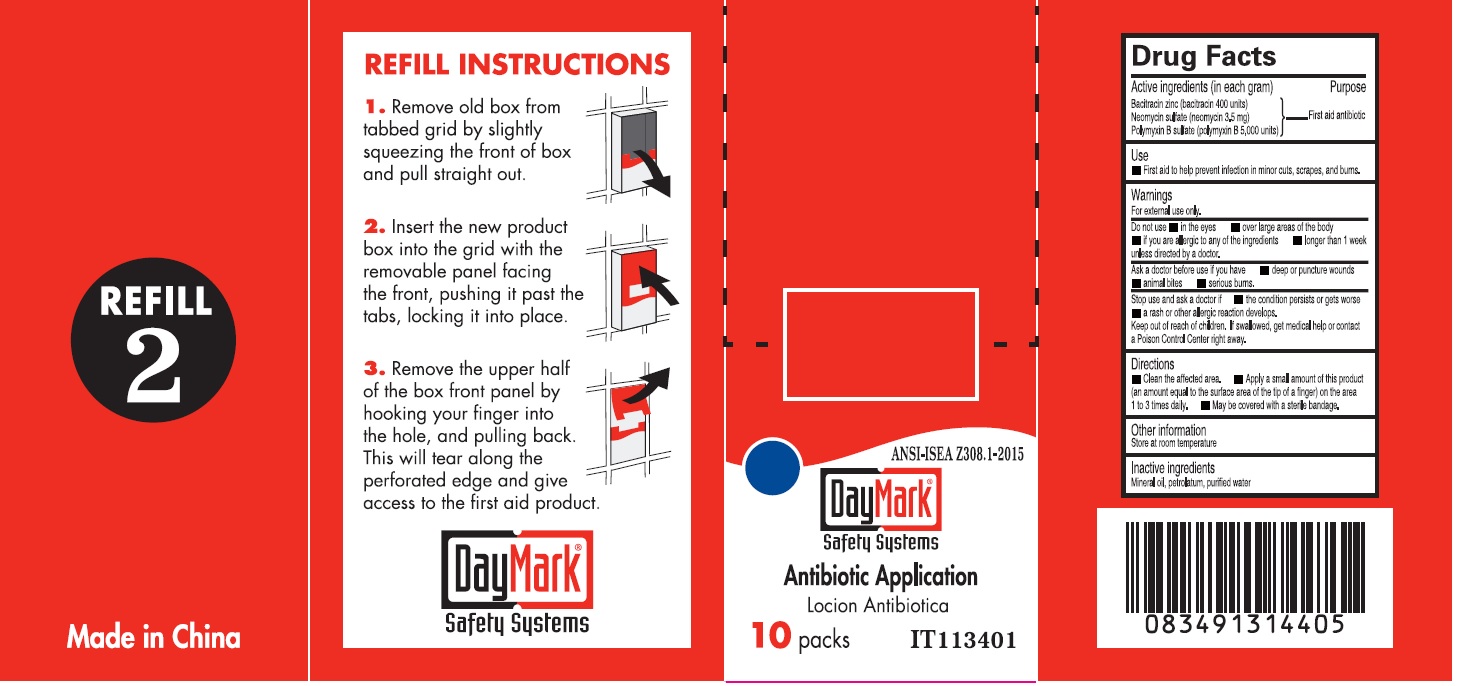
Drug Facts - Antibiotic Application
Active ingredients (in each gram)
Bacitracin zinc (bacitracin 400 units) Neomycin sulfate (neomycin 3.5mg) Polymyxin B sulfate (polymyxin B 5,000 units)
Purpose
First aid antibiotic
Use
- First aid to help prevent infection in minor cuts, scrapes, and burns.
Warnings
For external use only
Do not use
• in the eyes • over large areas of the body • if you are allergic to any of the ingredient • longer than 1 week unless directed by a doctor.
Ask a doctor before use if you have
• deep or punture wounds • animal bites • serious burns.
Stop use and ask a doctor if
• the condition persists or gets worse • a rash or other allergic reaction develops.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
• Clean the affected area. • Apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily. • May be covered with a sterile bandage.
Inactive ingredients
Mineral oil, petrolatum, purified water
Antiseptic Towelettes (49687-0016-0) Labeling:
Antibiotic Application (49687-0013-0) Labeling:
Refill 2 (49687-0016-0) Labeling:

CMC Group, Inc.


