Stop use and ask a doctor if
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
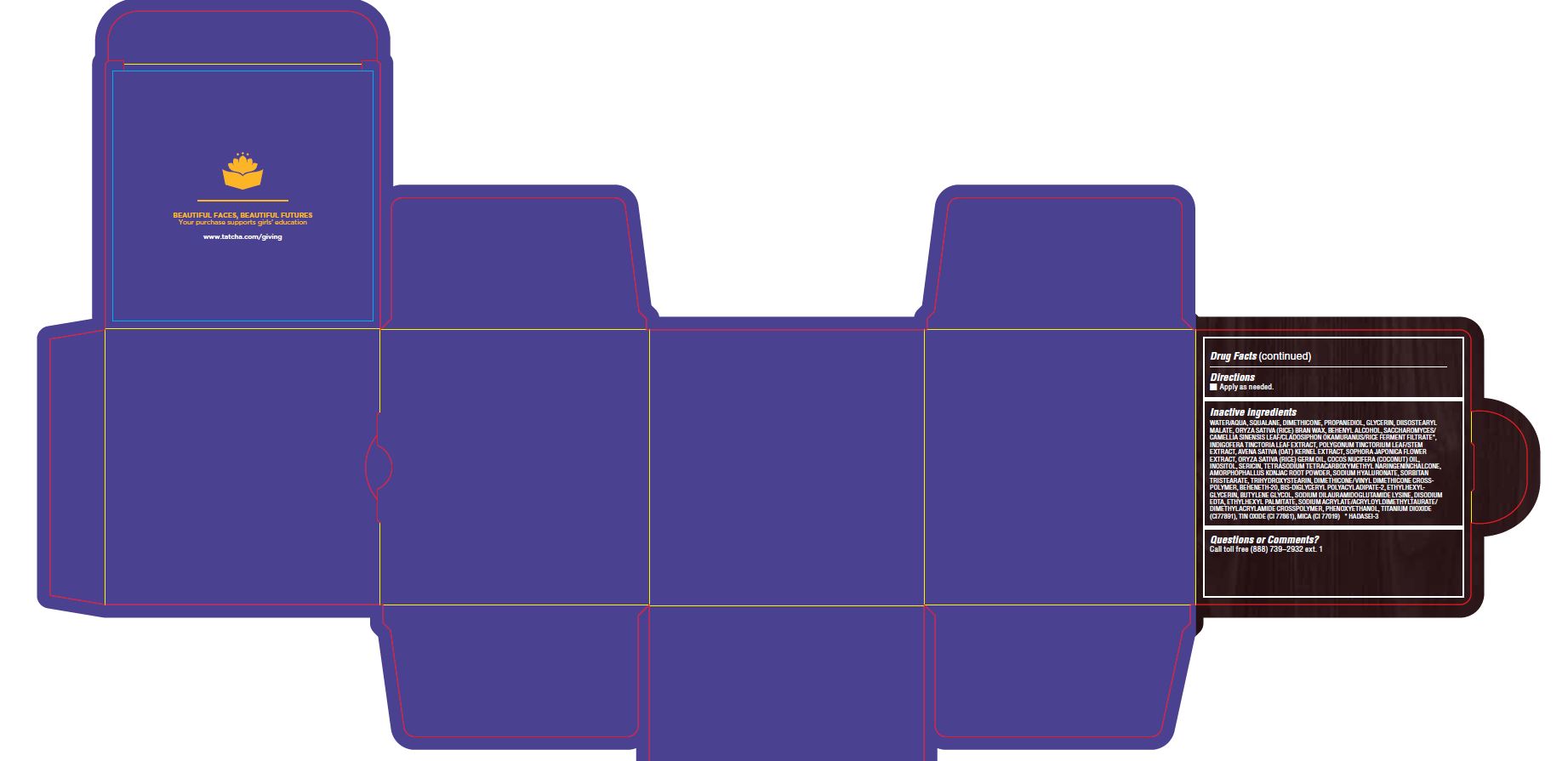
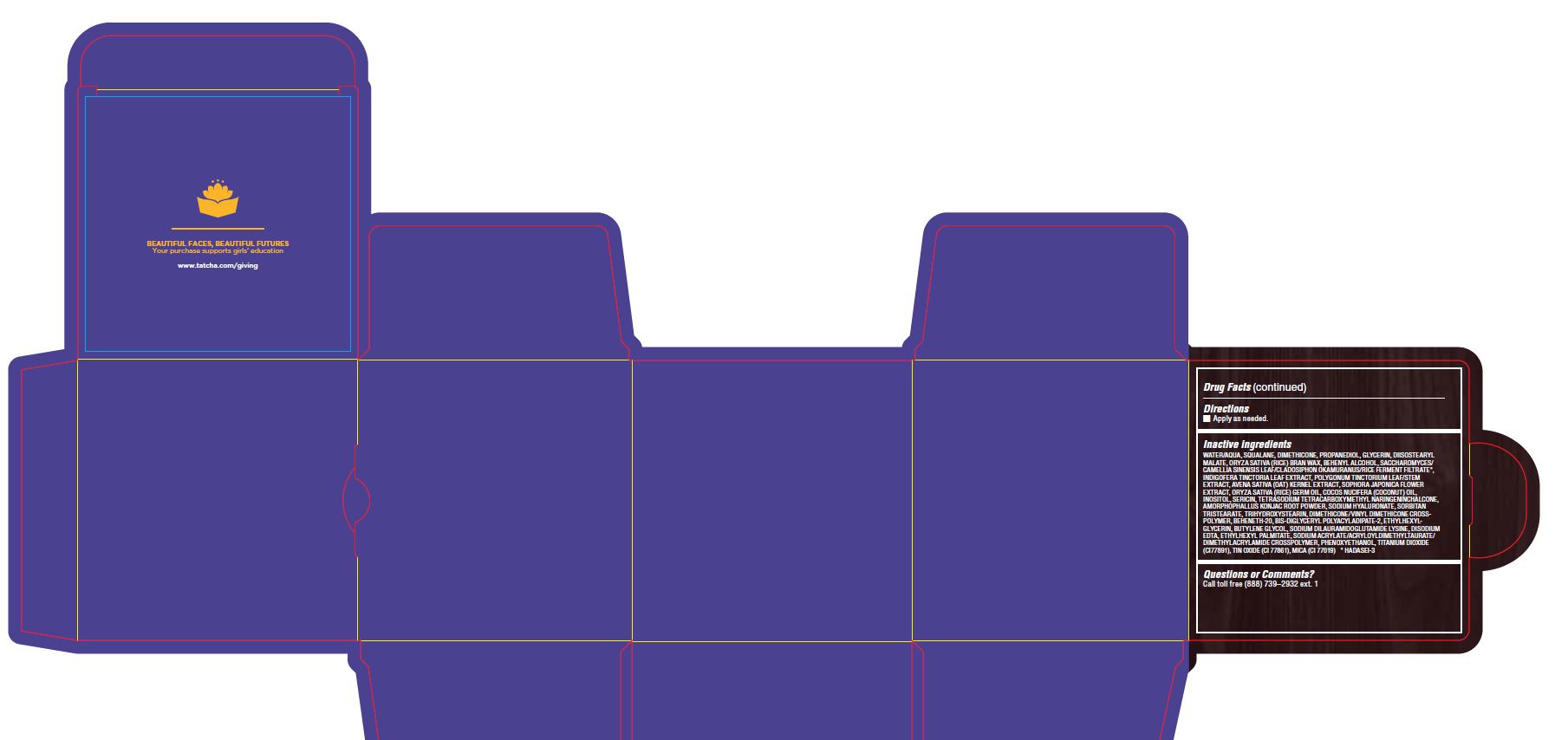
Inactive ingredients
WATER/AQUA, SQUALANE, DIMETHICONE, PROPANEDIOL, GLYCERIN, DIISOSTEARYL MALATE, ORYZA SATIVA (RICE) BRAN WAX, BEHENYL ALCOHOL, SACCHAROMYCES/CAMELLIA SINENSIS LEAF/CLADOSIPHON OKAMURANUS/RICE FERMENT FILTRATE*, INDIGOFERA TINCTORIA LEAF EXTRACT, POLYGONUM TINCTORIUM LEAF/STEM EXTRACT, AVENA SATIVA (OAT) KERNEL EXTRACT, SOPHORA JAPONICA FLOWER EXTRACT, ORYZA SATIVA (RICE) GERM OIL, COCOS NUCIFERA (COCONUT) OIL, INOSITOL, SERICIN, TETRASODIUM TETRACARBOXYMETHYL NARINGENINCHALCONE, AMORPHOPHALLUS KONJAC ROOT POWDER, SODIUM HYALURONATE, SORBITAN TRISTEARATE, TRIHYDROXYSTEARIN, DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER, BEHENETH-20, BIS-DIGLYCERYL POLYACYLADIPATE-2, ETHYLHEXYLGLYCERIN, BUTYLENE GLYCOL, SODIUM DILAURAMIDOGLUTAMIDE LYSINE, DISODIUM EDTA, ETHYLHEXYL PALMITATE, SODIUM ACRYLATE/ACRYLOYLDIMETHYLTAURATE/ DIMETHYLACRYLAMIDE CROSSPOLYMER, PHENOXYETHANOL, TITANIUM DIOXIDE (CI77891), TIN OXIDE (CI 77861), MICA (CI 77019) * HADASEI-3