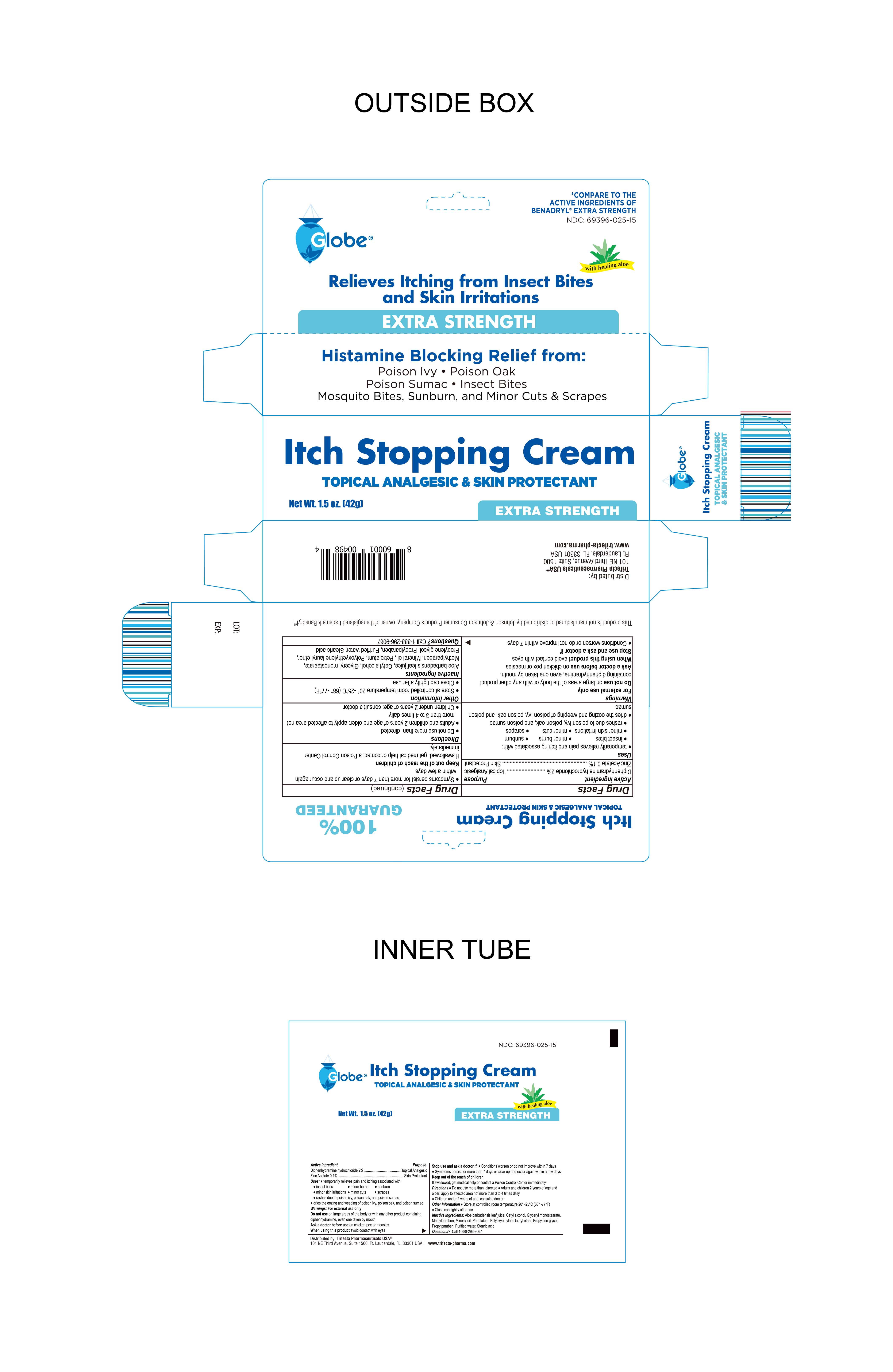
For the temporary relief from pain and itching associated with
- insect bites
- minor burns
- minor skin irritation
- rashes due to poison ivy, poison oak, and poison sumac
- dries the weeping and oozing of poison ivy, oak and sumac
Warnings
For External Use Only
Do not use on large areas of the body or with any other product containing diphenhydramine, even one taken by mouth
Ask a doctor before use on chicken pox or measles
When using this product avoid contact with eyes
Stop use and ask a doctor if
- Conditions worsen or do not improve within 7 days
- Symptoms persist for more than 7 days or clear up and occur again within a few days
Stop use and ask a doctor
- Conditions worsen or do not improve within 7 days
- Symptoms persist for more than 7 days or clear up and occur again within a few days
Keep out of the reach of children
If swallowed, get medical help or contact a Poison Control Center immediately
Directions
- Do not use more than directed
- Adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
- Children under 2 years of age: consult a doctor
Other information
- Store at controlled room temperature 20º-25ºC (68º-77ºF)
- Close cap tightly after use
Questions? Call 1-888-296-9067
Inactive Ingredients
Aloe Vera (Aloe Barbadensis) leaf juice, Cetyl alcohol, Glyceryl monostearate, Methylparaben, Mineral Oil, Petrolatum, Polyoxyethylene lauryl ether, Propylene glycol, Propylparaben, Purified Water, Stearic acid