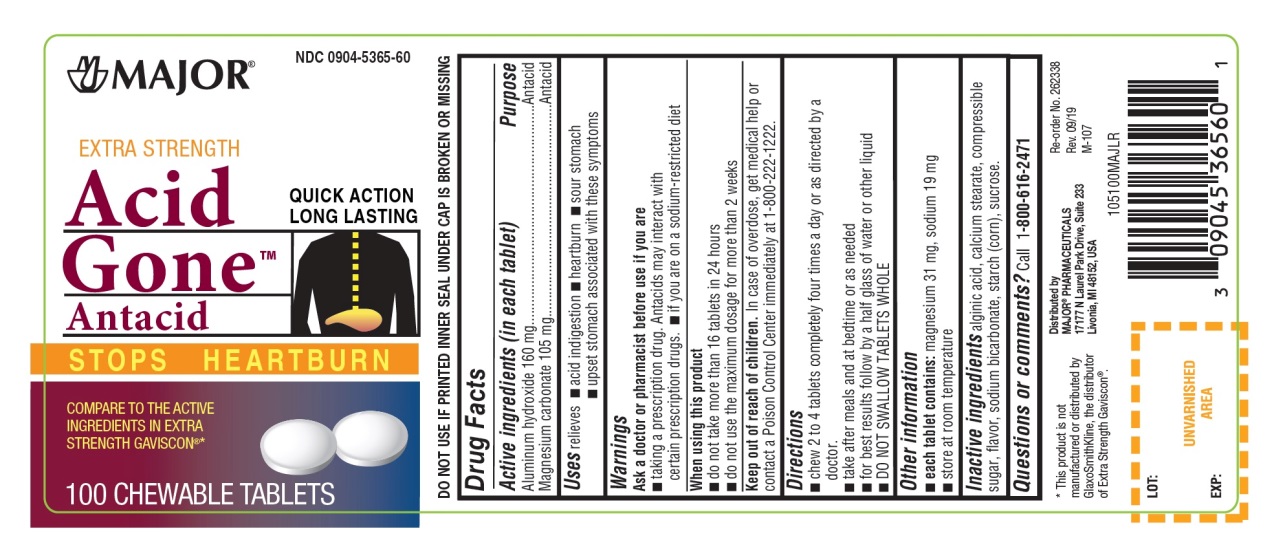
Uses
relieves
- ▪
- acid indigestion
- ▪
- heartburn
- ▪
- sour stomach
- ▪
- upset stomach associated with these symptoms
Warnings
Ask a doctor or pharmacist before use if you are
- ▪
- taking a prescription drug. Antacids may interact with certain prescription drugs.
- ▪
- if you are on a sodium-restricted diet
Directions
- ▪
- chew 2-4 tablets four times a day or as directed by a doctor
- ▪
- take after meals and at bedtime or as needed
- ▪
- for best results follow by a half glass of water or other liquid
- ▪
- DO NOT SWALLOW TABLET WHOLE
Inactive ingredients
alginic acid, calcium stearate, compressible sugar, flavor, sodium bicarbonate, starch(corn), sucrose.
Principal Display Panel
NDC 0904-5365-60
EXTRA STRENGTH
Acid Gone™
Antacid
STOPS HEARTBURN
QUICK ACTION
LONG LASTING
COMPARE TO THE ACTIVE INGREDIENT IN EXTRA STRENGTH GAVISCON®*
100 CHEWABLE TABLETS
Distributed by:
MAJOR® PHARMACEUTICALS
17177 N Laurel Park Drive, Suite 233
Livonia, MI 48150, USA
*This product is not manufactured or distributed by GlaxoSmithKline, the distributor of Extra Strength Gaviscon®