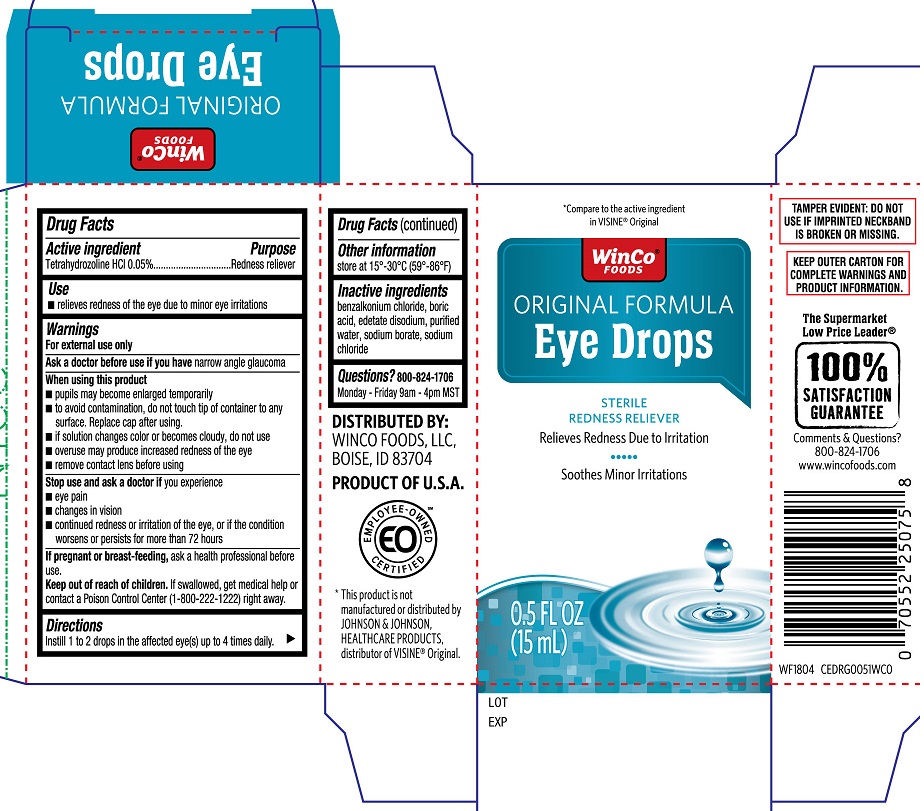
Active ingredient
Tetrahydrozoline HCL 0.05%
Uses
- relieves redness of the eye due to minor eye irritations
Warnings
For external use only
Ask a doctor before use if you have
narrow angle glaucoma
When using this product
- pupils may become enlarged temporarily
- to avoid contamination, do not touch tip of container to any surface. Replace cap after using
- if solution changes color or becomes cloudy, do not use
- overuse may produce increased redness of the eye
- remove contact lens before using
Stop use and ask a doctor if
you experience
- eye pain
- changes in vision
- continued redness or irritation of the eye, or if the condition worsens or persists for more than 72 hours
If pregnant or breast-feeding
ask a health professional before use
Keep out of reach of chlidren
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
Instill 1 or 2 drops in the affected eye(s) up to four times daily
Other information
store at 15-30°C (59°-86°F)
Inactive ingredients
benzalkonium chloride, boric acid, edetate disodium, purified water, sodium borate, sodium chloride