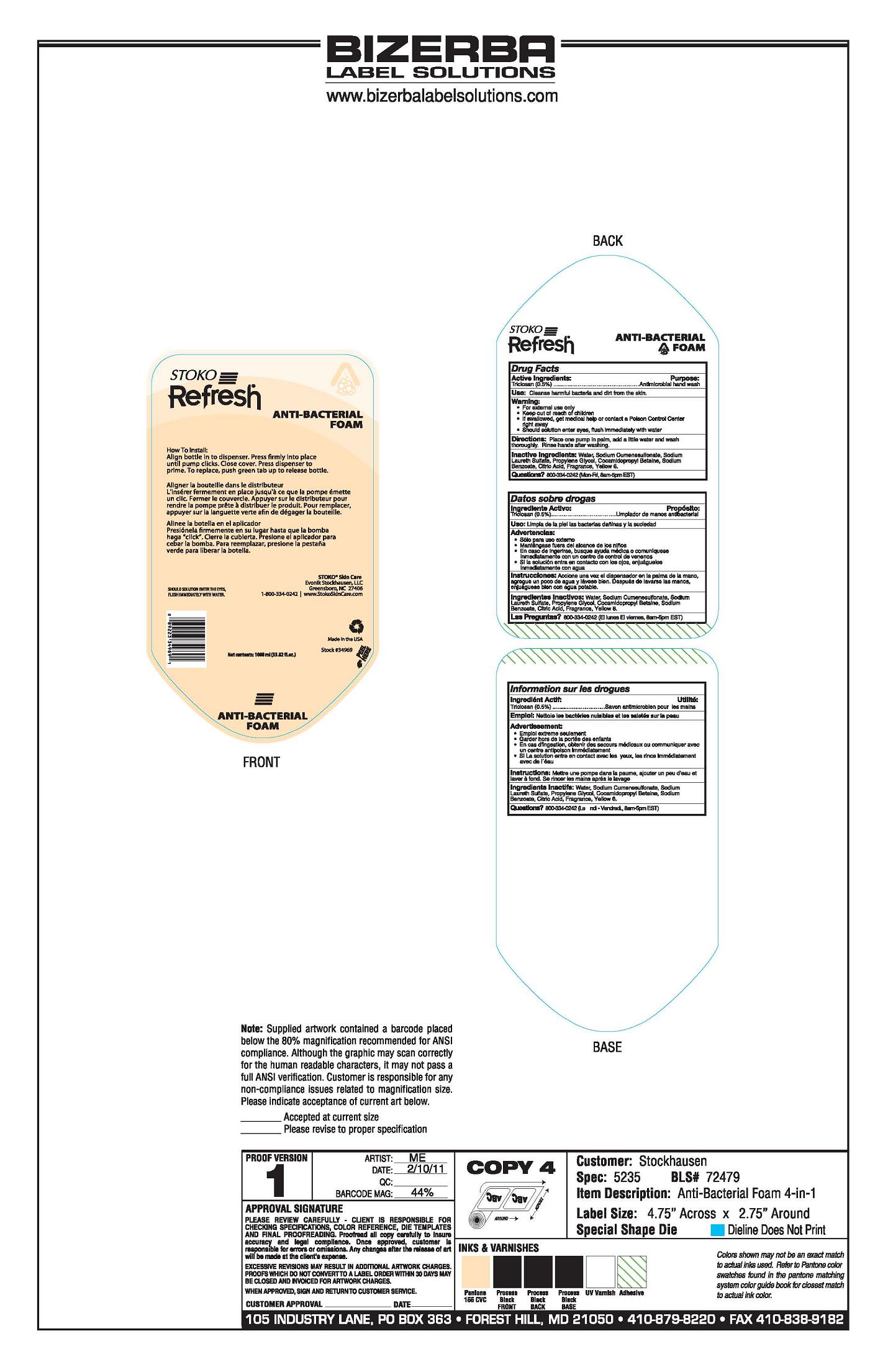
Warning
- For external use only
- If swallowed, get medical help or contact a Poison Control Center right away
- Should solution enter eyes, flush immediately with water
Directions
Place one pump in palm, add a little water and wash thoroughly. Rinse hands after washing.
Inactive Ingredients
Water, Sodium Cumenesulfonate, Sodium Laureth Sulfate, Propylene Glycol, Cocamidopropyl Betaime, Sodium Benzoate, Citric Acid, Fragrance, Yellow 6.
Principal Display Panel
Stoko
Refresh
Anti-Bacterial Foam
How To Install:
Align bottle in to dispenser. Press firmly into place
until pump clicks. Close cover. Press dispenser to
prime. To replace, push green tab up to release bottle.
Aligner la bouteille dans le distributeur
L'inserer fermement en place jusqu'a ce que la pompe emette
un clic. Fermer le couvercle. Appuyer sur le distributeur pour
rendre la pompe prete a distribuer le produit. Pour remplacer,
appuyer sur la languette verte afin de degager la bouteille.
Alinee la botella en el aplicador
Presionela firmemente en su lugar hasta que la bomba
haga "click". Cierre la cubierta. Presione el aplicador para
cebar la bomba. Para reemplazar, presione la pestana
verde para liberar la botella.
Should Solution Enter The Eyes
Flush Immediately With WaterStoko Skin Care
Evonik Stockhausen, LLC
Greensboro, NC 27406
1-800-334-0242 I www.StokoSkinCare.com
Made in the USA
Stock#34969
Net contents: 1000 ml (33.82 fl. oz.)